Information
Journal Policies
Using Emulsigen®-D as Recent Adjuvant in Trivalent Foot and Mouth Disease Vaccine
Walaa Shabana; Ismail, A. Fathy,A; Hind Mohamed and Mossad, W
Copyright :© 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The immunity and protective capability produced by vaccines can vary remarkably according to the kinds of adjuvant being used. Through this work three formulae of the inactivated trivalent FMD vaccine (O pan Asia, A Iran O5, and SAT2 / EGY/2012) were prepared using different adjuvants including Emulsigen®- D; Montanid ISA 206 and Emulsigen®-D (ED) with aluminum hydroxide gel (ALOH). All of these vaccine formulae were found to be free from foreign contaminants and safe. Also, each vaccine formula was injected in a separate sheep group and serum samples were collected along 38-week post-vaccination for tracing of antibodies against FMDV serotypes by serum neutralization test (SNT) and enzyme-linked immune sorbent assay (ELISA). Results of SNT and ELISA revealed that the onset of protective antibody titer was achieved early in the Emulsigen® and Emulsigen® with ALOH gel vaccinated groups as it starts at 2nd -week post-vaccination while the onset of protective antibody titer in Montanide ISA 206 vaccinated group started at 3rd-week post-vaccination. Concerning the highest peak antibody titer values were induced by Emulsigen®-D with aluminum hydroxide gel on 8th-week post- vaccination followed by Emulsigen®-D on 10th-week post-vaccination and lastly for Montanid ISA 206 on 12th-week post-vaccination. Concerning the duration of protective immunity against the three serotypes of FMDV included in the vaccine, the results revealed that the longest duration was achieved by the Emulsigen® D alone and with the ALOH adjuvanted vaccines as it lasts for 36-week post vaccination as recorded by the SNT values. The Montanide ISA 206 adjuvanted vaccine group protective SNT antibody titer against the three serotypes lasts for 32- weeks post vaccination. Depending on these findings, it could be concluded that Emulsigen®-D and with aluminum hydroxide gel induce the superior immune response of sheep to the trivalent FMD vaccine over the Montanide ISA 206 adjuvanted trivalent FMD vaccine.
FMD; SNT; ELISA; Emulsigen®-D; Montanid ISA 206,Animal and Veterinary Sciences
1. Introduction
Foot-and-mouth disease (FMD) is a viral infectious disease that forms vesicles in the mouth and hooves of artiodactyls, such as pigs, cattle, sheep, and goats resulting in weight loss, reduced milk production and growth delays. The disease can be spread rapidly not only by the excrement of infected animals but also by contaminated feed, vehicles, and humans. Efforts directed to the eradication and prevention of FMD centering on stamping-out policies are controversial and the prevention, and control of the disease using vaccines have become areas of extreme interest Min-Eun et al 2016. Thus, the economic damage is substantial once an outbreak occurs. Therefore, FMD is subject to international regulations for the global trade of both livestock and their products Kitching 1999, Meyer and Knudsen 2001. The administration of vaccines is a highly effective method for preventing FMD.
The causative agent is the FMD virus which has seven serological types identified as O, A, C, SAT1, SAT2, SAT3, and Asia1 Doel and Baccarini 1981, Barnett and Carabin 2002. FMD is characterized by fever, lameness and vesicular lesions on the feet, tongue, snout, and teats with high morbidity and low mortality Satya 2009. The disease is enzootic in Egypt, with many outbreaks having been reported since 1950. The present serotypes of FMD virus in Egypt now are SAT2, A and O. Serotype O was lastly reported Aidaros 2002. Serotype A was firstly recorded in Egypt in 2006 through importation of live animals and resulted in sever clinical signs in cattle and buffaloes Abd El- Rahman et al 2006. The recent FMDV serotype introduction is the serotype SAT2 in 2012, also from the importation of live animals. All these serotypes were isolated and typed by Veterinary Serum and Vaccine Research Institute (VSVRI) and confirmed by World Reference Laboratory (WRL) for FMD, Pirbright Institute, United Kingdom Abd El- Aty et al 2013. Vaccination is the corner stone and effective method for preventing FMD. The selection of an appropriate adjuvant is the most important factor in determining the efficacy of potent vaccines to ensure a protective immunity enables susceptible animals to withstand the disease outbreaks Min-Eun et al 2016.
Emulsigen®-D is an oil-in-water emulsion contains uniformly dispersed micron-size oil droplets, which ensure maximum emulsion stability and decreased viscosity. Micron-size oil droplets also increase the surface area available to antigens, reducing the quantity of oil required in the final produced vaccine. Emulsigen®-D reduces the undesirable side effects associated with other oil-in-water or water-in-oil adjuvants while eliciting a rapid and strong immune response Technologies M. Emulsigen®-D Technical Bulletin 2012.Emulsigen®-D as an adjuvant produces increased immunogenicity because it incorporates dimethyl-dioctadecyl ammonium bromide (DDA), which is a T-cell immune stimulator in Emulsigen®. Its efficacy as an adjuvant was proved in Toxoplasma gondii and rabies Hiszczynska-Sawicka et al 2010 and Kaur et al 2010. According to Kaur et al 2010 the DDA contained in Emulsigen®-D induces enhancement of immune responses by increasing the surface area of antigens in oil-in-water emulsions so that antigen spread slowly. Therefore, protection against Aujeszky’s disease virus is increased when infected animals have been vaccinated with Emulsigen® plus DDA. Also, aluminum compounds have been known to be the most frequently used adjuvant in veterinary vaccines Gupta 1998. These compounds have been found to induce memory cell responses and long-lasting protection when animals have been inoculated with vaccines, thereby enhancing immune reactions Rimaniol et al 2004. Among them, aluminum phosphate and aluminum hydroxide are the only adjuvants approved for routine use in humans because of their relatively low toxicity Li and Nookala 2007.
In this study, we evaluate comparatively the efficacy of experimental batches of FMD trivalent vaccine (including O pan Asia, A Iran O5 and SAT2 / EGY/2012) using various adjuvants as Emulsigen®- D alone, and with Aluminium hydroxide gel and Montanide ISA 206 aiming to determine the best vaccine formula is having the optimum antigenicity and immunogenicity. The efficacy of prepared vaccine formulae will be tested in dairy sheep as one of the susceptible animal species for FMD.
2. Material And Methods
The experiment was carried out according to the protocol of the Institutional Animal Ethics Committee, and the authors had permission of the animal owners at the private farms.
Local Foot and Mouth disease virus serotypes O pan Asia, A Iran O5 and SAT2 / EGY/2012 propagated in Baby Hamster Kidney (BHK21) cell line monolayer which was supplied by the Department of Foot and Mouth Diseases Research, Veterinary Serum and Vaccine Research Institute. The titer of the three serotypes was expressed as log 10TCID50/ml as described by Reed and Muench 1938 and the complement fixation test was carried out according to Health Protection Agency 2009 These viruses were used for the preparation of trivalent inactivated vaccine as well as in serological tests.
a. Sheep
Twenty native breed sheep in a private farm free from FMD antibodies as screened by serum neutralization test were divided into four groups (5 animals/group). Each of 3 experimental FMD trivalent vaccines adjuvanted with Emulsigen® D, Emulsigen® D with ALOH, Montanide ISA 206, was inoculated as each in a sheep group keeping one group without vaccination as a negative control. The vaccine dose was 1.5 ml/animal inoculated subcutaneously where each dose contains 109 TCID50 of each type of Foot and mouth disease virus serotype.
b. Suckling Baby Mice
Suckling Swiss baby mice, two to four days old, (Charles River Strain, USA) were used for testing the safety of the inactivated viruses according to OIE 2017.
Serum samples were obtained from all sheep groups at the time of vaccination (zero time) then every week till four weeks, every two weeks for 16 weeks, every four week till 32 weeks post vaccination and lastly every two weeks till the end of the experiment (38 -weeks post vaccination). These samples were subjected for estimation of FMD antibodies in vaccinated animals using SNT and indirect ELISA.
Baby Hamster kidney cell line (BHK21) was supplied by Veterinary Serum and Vaccine Research Institute, Abbasia, Cairo using Eagle’s medium supplemented with 8-10% bovine serum as described by Xuan et al 2011 and used for application of serum neutralization test, virus titration, and vaccine preparation.
Each FMD virus serotype (O, A and SAT2) at the 7th passage on BHK monolayer was treated with chloroform at a concentration of 1.5% (Volume / Volume) as a clarification method before inactivation. Inactivation was occurred using combination 1mM of BEI and 0.04% FA (BEI-FA) according to the method described by Barteling and Cassim 2004 and Ismail et al 2013. Sodium thiosulphate 20% in final concentration of 2% and sodium bisulphite 20% in final concentration of 2% were added after the inactivation process to neutralize the excess of BEI and formalin residues.
The antigens were added to each of the following adjuvants:
1. Emulsigen®-D (EMULSIGEN®-D; MVP Technologies, NE, USA)
2. ISA 206(MONTANIDETMISA 206 VG; SEPPIC, France)
3. Emulsigen®-D with aluminum hydroxide gel (Rehydragel®HPA; General Chemical, NJ, USA).
The ratio of adjuvant to total volume was 20:80 for Emulsigen®-D as recommended by Technologies M. Emulsigen®-D Technical Bulletin 2012 and Min-Eun et al 2016 and was 50:50 for ISA 206(volume (v/v) as mentioned by El-Sayed et al.2015. For the oil/gel adjuvant mixture, we added 10% aluminum hydroxide gel. The mixture was stirred at 300 rpm for10 min at 30◦C in a water incubator to form a water-in-oil-in-water blend.
The prepared vaccine batches were tested for their freedom of aerobic and anaerobic bacteria; fungal and mycoplasma contaminants where vaccines samples were cultured on thioglycolate broth, Sabouraud's, Nutrient agar; phenol dextrose media and mycoplasma medium. The safety of the prepared vaccines was done in baby mice according to OIE 2017.
Serum samples collected from the vaccinated sheep were tested for monitoring of the exhibited FMD antibody titers against the three serotypes by serum neutralization test (SNT) using the technique described by Ferreira 1976 and indirect enzyme-linked immune sorbent assay (ELISA) according to Voller et al 1976.
3. Results And Discussion
The control of FMD is dependent on the vaccination of susceptible animal species with inactivated whole virus vaccines Rodriguez and Grubman 2009. Vaccination with good quality FMD vaccines helps in the prevention of livestock production losses and reduces the overall incidence of the disease Hunter 1998. The selection of adjuvant in FMD vaccine formulation is important for both early and long-lasting immunity and protection. Hence, efforts are focused on developing adjuvant that can promote protective immunity through induction of enhanced and more durable antibody responses Dar et al 2013.
Attention is often directed to improve the potency of FMD vaccine aiming to provide the highest immune level in vaccinated animals to be able to withstand virus infection and accordingly avoid the suggested dramatic economic losses.
Emulsigen®-D is a unique oil-in-water emulsion and contains uniformly dispersed micron- size oil droplets. These Micron-size oil droplets increase the surface area available to antigens, reducing the quantity of oil required in the final vaccine.
Emulsigen®-D incorporates dimethyl-dioctadecyl ammonium bromide (DDA) which is a T-cell immune stimulator. According to Kaur et al. 2010, the DDA contained in Emulsigen®-D induces the enhancement of immune responses by increasing the surface area of antigens in oil-in-water emulsions so that antigens spread slowly. The use of ALOH gel in combination with oil is attributed as it is the most commonly used adjuvant in commercial vaccines Rimaniol et al 2004 and a previous report showed that AL induces Th2-type responses in animal models, facilitating the dissemination of antibodies from the injected region Gupta et al 1995 and Brewer et al 1996. Also, the gel was shown to play an important role in memory responses by inducing the differentiation of macrophages Min-Eun et al 2016. The combined components of oil and AL have been used to protect against rabies in bovines Reddy, and Srinivasan 1997. So in this study, we apply the use of Emulsigen® -D and the use of ALOH gel in combination with oil as an adjuvant in foot and mouth disease vaccine and tracing the humeral immune response of sheep upon using these adjuvants.
This work deals with three prepared formulae of inactivated trivalent FMD vaccine (O pan Asia, A Iran O5 and SAT2 / EGY/2012) were prepared using three different adjuvants including Emulsigen®-D; Montanid ISA 206 and Emulsigen®-D with aluminum hydroxide gel The present obtained results revealed that all the prepared FMD trivalent vaccine formulae are free from foreign contaminants and safe inducing no abnormal post vaccination signs in vaccinated sheep in agreement with what recommended for such vaccine OIE 2017.
The antibody titer against the three serotypes (O, A and SAT2) were monitored in the serum samples using the serum neutralization and ELISA tests. Before vaccinating the different sheep groups, we ensure that all sheep involved in the experiment are free from antibody titer against the foot and mouth disease virus.
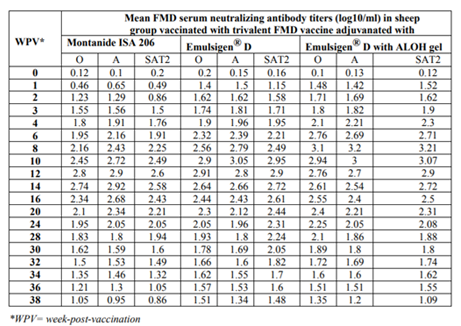
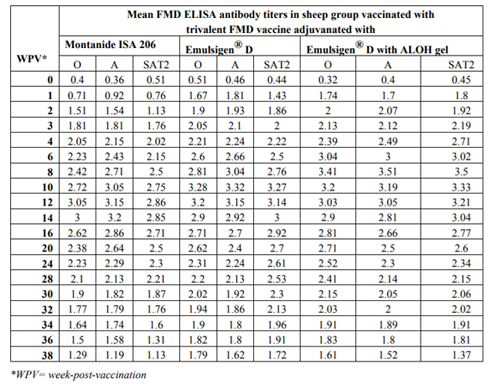
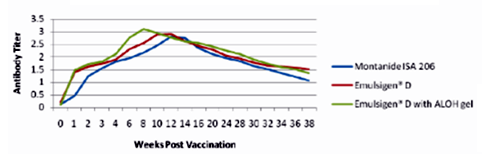
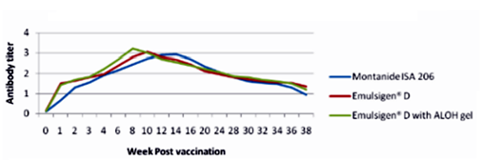
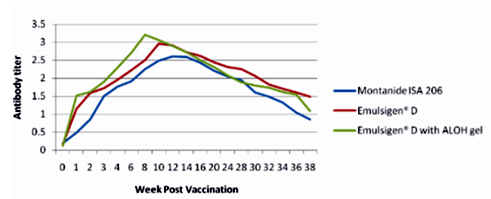
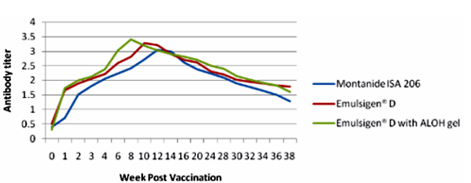
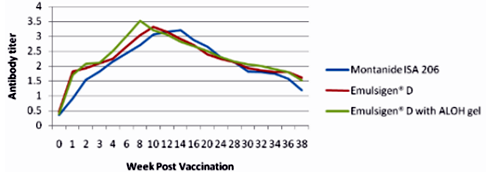
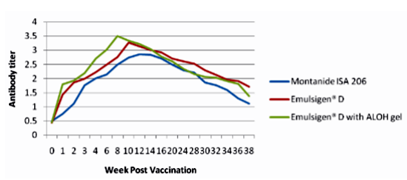
The results as tabulated in tables no. (1&2) and demonstrated by the figures (1-6) revealed that the onset of protective antibody titer was achieved early in the Emulsigen® and Emulsigen® with ALOH gel vaccinated groups as it starts at 2nd -week post vaccination while the onset of protective antibody titer in Montanide ISA 206 vaccinated group started at 3rd-week post- vaccination. Concerning the highest peak antibody titer values were induced by Emulsigen®-D with aluminum hydroxide gel on 8th-week post-vaccination (3.1; 3.2 &3.21 log10 for serotypes O, A &SAT-2 respectively); followed by Emulsigen®-D on 10th-week post-vaccination (2.9; 3.05& 2.95 log10 for type O, A &SAT-2 respectively) and then for Montanid ISA 206 on 12th - week post-vaccination (2.8; 2.9 and 2.6 log10 for type O, A &SAT-2 respectively) as evaluated by SNT. Concerning the duration of protective immunity against the three serotypes of FMDV included in the vaccine, the results revealed that the longest duration was achieved through the Emulsigen® D alone and with the ALOH adjuvanted vaccine as it lasts for 36 weeks post- vaccination as recorded by the SNT values. The Montanide ISA 206 adjuvanted vaccine group protective SNT antibody titer against the three serotypes lasts for 32 weeks post-vaccination. So from these results there is a two weeks protection duration difference between the different vaccinated groups. ELISA results as a confirmatory test came in a parallel manner with those results obtained by SNT. From these results, it is clear that the use of Emulsigen® D adjuvant and, the addition of ALOH gel have a positive impact on the onset, peak and duration of protective immunity.
The previous results come in parallel with that obtained by Min-Eun et al 2014 as he mentioned that a high level of neutralizing antibodies in the ED + AL or ISA 201 groups exhibited a statistically significant difference from that in the ISA206 group. Regarding cell- mediated immune responses, the ED and ED + AL vaccination groups exhibited statistically significant increases after antigen stimulation in both Th1 and Th2 cytokines, although they exhibited a low level of cytokines. Th1 reactivity was stronger in the ED + AL vaccination group than the ED-only vaccination group. Also, he found that a high level of neutralizing antibodies developed in a short period in the group of dairy goats inoculated with combined ED + AL, proving that Emulsigen®-D in combination with aluminum hydroxide enhances the immune response in both pigs and dairy goats against foot and mouth disease virus.
In conclusion for the present work we found that the use of Emulsigen® D in sheep has an improvement immunogenicity effect over the use of the Montanide ISA 206 and also the use ALOH in combination potentiate the effects of ED adjuvants in the trivalent FMD vaccine.
References
- Min-Eun Park , Seo-Yong Lee , Rae-Hyung Kim, Mi-KyeongKo , Jeong-Nam Park , Kwang-Nyeong Lee, Su-Mi Kim , Joo-Hyung Choi , Su-Hwa You , Byounghan Kim , Jong-Soo Lee , Jong-Hyeon Park ( 2016) : Altered adjuvant of foot-and-mouth disease vaccine improves immune response and protection from virus challenge. Trials in Vaccinology 5 (2016) 97– 104.
- Kitching R.P. Foot-and-mouth disease: current world situation, Vaccine 17 (1999) 1772–1774.
- Meyer R.F., Knudsen R.C., Foot-and-mouth disease: a review of the virus and the symptoms, J. Environ. Health 64 (2001) 21–23.
- Doel TR, Baccarini PJ (1981) Thermal stability of foot-and-mouth disease virus. Arch Virol 70: 21–32.
- Barnett PV, Carabin H (2002) A review of emergency foot-and-mouth disease (FMD) vaccines. Vaccine 20: 1505–14.
- Satya, P. (2009) Vaccination against foot-and-mouth disease virus: Strategies and effectiveness. Expert Rev. Vaccines., 8(3): 347-365.
- Aidaros, H.A. (2002) Regional status and approaches to control and eradication of FMD in the middle east and North Africa. Rev. Sci. Tech. Off. Int. Epizoot., 21(3): 451-458
- Abd El- Rahman, A.O., Farag, M.A., El- Kilany, S., Ali, S.M. and Yazed, M.A. (2006) Isolation and identification of serotype O of foot and mouth disease virus from imported bulls and its correlation to the current used vaccine strain O1/3/1993. Proceeings of 3rd International Conference Vetrinary Research Division., NRC, Cairo, Egypt. p91-100.
- Abd El-Aty, S.M., Fakry, H.M., Hind, M.D., El-Sayed, E.I., Wael M.G., Rizk, S.A., Abu-Elnaga, H., Mohamed, A.A., Abd El-kreem, A. and Farouk E.M. (2013) Isolation and Molecular characterization of foot and mouth disease sat2 virus during outbreak 2012 in Egypt. J. Vet. Adv., 3(2): 60-68.
- Technologies M. Emulsigen®®-D Technical Bulletin; 2012.
- EL-Sayed E. M , Gamal W. M, Hassan A.I, Mahdy S.E, Hegazy A.Z, ABDEL-ATTYM.M 2015. Comparative study on the immunopotentiator effect of ISA 201, ISA 61,ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine, Veterinary World 8(10): 1189-1198. doi: 10.14202/vetworld.2015.1189-1198
- Hiszczynska-Sawicka E, Li H, Xu JB, Oledzka G, Kur J, Bickerstaffe R, et al. Com- parison of immune response in sheep immunized with DNA vaccine encoding Toxoplasmagondii GRA7 antigen in different adjuvant formulations. Exp Para-sitol 2010;124:365–72.
- Kaur M, Saxena A, Rai A, Bhatnagar R. Rabies DNA vaccine encoding lysosome- targeted glycoprotein supplemented with Emulsigen®-D confers complete protection in preexposure and postexposure studies in BALB/c mice. FASEBJ: Off Publ Fed Am SocExpBiol 2010;24:173–83.
- Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev1998;32:155–72.
- Rimaniol AC, Gras G, Verdier F, Capel F, Grigoriev VB, Porcheray F, et al. Alu-minum hydroxide adjuvant induces macrophage differentiation towards aspecialized antigen-presenting cell type. Vaccine 2004;22: 3127–35.
- Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 andinduce IL-1beta and IL-18 release. J Immunol 2007;178:5271–6.
- Reed, L.J. and Muench, H. (1938) A simple method of estimating 50% end points. Am. J. Hyg., 27: 493-497.
- Health Protection Agency. (2009) Complement fixation tests. Issue No.: 3 Issue date 11.12.09 Issued by: Standards Unit, Department for Evaluations, Standards and Training page 1 of 23.
- Xuan, H., Li, Y., Fang, H. and Zheng, C. (2011) Establishment of persistent infection with foot and mouth disease virus in BHK21 cells. Virol. J., 8: 169.
- Barteling, S.J. and Cassim, N.I. (2004) Very fast (and safe) inactivation of FMD virus and enteroiruses by a combination of binary ethylenimine and formaldehyde. Schudel, A., Lombard, M. editors. Control of Infections Animal Disease by Vaccination. Vol.119. Kager, Basel. p449-455.
- Ismail, A.H., El-Mahdy, S.A., Mossad, W.G., Abd El-Krim, A.S., Abou El-Yazid, M. and Ali, S.M. (2013) Optimization of the inactivation process of FMD virus serotype SAT-2 by Binary Ethyleneimine (BEI). J. Vet. Adv., 3(3): 117-124.
- OIE (2017): OIE/FAO Foot-and-Mouth Disease Reference Laboratory Network, Annual Report 2017.
- FerreiraMEV(1976):Prubademicro neutralization poraestudies de anticueropos de la fibreaftosa. 13th Centropanamericano Fibreaftosa, (21/22): 17-24.
- Voller, A., Bidwell, D. and Bartlett, A. (1976) Microplate enzyme immunoassay for the immunodignosis of virus infection. Am. Soc. Microbiol, 506-512.
- Rodriguez LL, Grubman MJ. Foot and mouth disease virus vaccines. Vaccine2009;27(Suppl. 4):D90–4.[16] Hunter P. Vaccination as a means of control of foot-and-mouth disease in sub-saharan Africa. Vaccine 1998;16:261–4.
- Hunter P. Vaccination as a means of control of foot-and-mouth disease in sub-saharan Africa. Vaccine 1998;16:261–4.
- Dar P, Kalaivanan R, Sied N, Mamo B, Kishore S, Suryanarayana VV, et al. Mon-tanide ISA 201 adjuvanted FMD vaccine induces improved immune responsesand protection in cattle. Vaccine 2013;31:3327–32.
- Hiszczynska-Sawicka E, Li H, Xu JB, Oledzka G, Kur J, Bickerstaffe R, et al. Com- parison of immune response in sheep immunized with DNA vaccine encodingToxoplasma gondii GRA7 antigen in different adjuvant formulations. Exp Para-sitol 2010;124:365– 72.[21]
- Kaur M, Saxena A, Rai A, Bhatnagar R. Rabies DNA vaccine encoding lysosome- targeted glycoprotein supplemented with Emulsigen®-D confers completeprotection in preexposure and postexposure studies in BALB/c mice. FASEBJ: Off Publ Fed Am Soc Exp Biol 2010;24:173–83.
- Gupta R.K., B.E. Rost, E. Relyveld, G.R. Siber, Adjuvant properties of aluminum and calcium compounds, Pharm. Biotechnol. 6 (1995) 229– 248.
- Reddy, V.A. Srinivasan, Performance of aluminium hydroxide gel and oil adjuvant rabies vaccines in bovines, Zentralbl Bakteriol 286 (1997) 523–526.
- Brewer J.M., M. Conacher, A. Satoskar, H. Bluethmann, J. Alexander, In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to Freund’s complete adjuvant, but continues to induce T helper 2 cytokine production, Eur. J. Immunol. 26 (1996) 2062–2066.
- Min-Eun Park, Seo-Yong Lee , Rae-Hyung Kim, Mi-KyeongKo ,Kwang-Nyeong Lee, Su-Mi Kim, Byoung-Kwan Kim , Jong-Soo Lee ,Byounghan Kim , Jong-Hyeon Parka (2014).Enhanced immune responses of foot and mouth disease vaccine using new oil/gel adjuvant mixtures in pigs and goats. Vaccine 32 (2014) 5221–5227.