Information
Journal Policies
Assessment and Control of Endotoxin Contamination in Baby Hamster Kidney Tissue Culture
Assem A Mohamed Wael Mosad*, Akram Zakria, Ehab El said, Ahmed Fathy, Amr I Hassanin, Mohamed A Gamil, and Hiam M Fakhry
Copyright :© 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: The presence of endotoxin in the products for injection can result in pyrogenic responses ranging from fever and chills, to irreversible and fatal septic shock.
Objective: Screening and control the endotoxin levels during propagation of Baby Hamster Kidney (BHK) tissue culture used in the preparation of foot and mouth disease (FMD) vaccine in veterinary serum and vaccine research institute (VSVRI) .
Method: This study was conducted by in vitro measuring endotoxin levels in the different ingredients used in BHK cells propagation using Limulus Amebocyte Lysate (LAL) test. Tested ingredients are water, sera, media and additives, cell culture reagents, glassware and plastic ware. Also the study evaluates the effect of different levels of endotoxin (50-1000 Endotoxin Units (EU)/ml) on the BHK cells quality (morphological abnormalities, growth and cells sensitivity to different FMD virus strains) and the effect of Polymyxin B Sulfate (PMB) as anti-endotoxin candidate.
Results: Our results show that high endotoxin levels (≥500 EU/ml) in BHK cells propagation procedures could minimize the quality of cells.
Conclusion: Finally we recommend using endotoxin free ingredients in the BHK tissue culture procedures in FMD vaccine preparation, Also Polymyxin B Sulfate could be a good anti-endotoxin candidate.
BHK, FMD vaccine, LAL test, EU, PMB
1. Introduction
Endotoxins are lipopolysaccharides (LPS) derived from cell membrane of Gram-negative bacteria and are responsible for its organization and stability Morrison et al., 1994, Raetz 1990, Rietschel and Brade 1992, Roslansky et al., 1991, Weary and Pearson 1988. In pharmaceutical industries it is possible to find endotoxins during production processes or in the final product. Although endotoxins are linked within the bacterial cell wall, they are continuously liberated into the environment. The release does not happen only with cell death but also during growth and division. Since bacteria can grow in nutrient poor media, such as water, saline, and buffers, endotoxins are found almost everywhere. A single Escherichia coli contains about 2 million LPS molecules per cell. Endotoxin elicits a wide variety of pathophysiological effects. In conditions where the body is exposed to LPS excessively or systemically (as when small concentrations of LPS enter the blood stream), a systemic inflammatory reaction can occur, leading to multiple pathophysiological effects, such as endotoxin shock, tissue injury, and death (Anspach 2001, Erridge et al., 2002, Ogikubo et al., 2004). However, endotoxin does not act directly against cell or organs but through activation of the immune system, especially through monocytes and macrophages, with the release of a range of pro-inflammatory mediators, such as tumor necrosis factor (TNF), interleukin (IL)-6 and IL-1. Pyrogenic reactions and shock are induced in mammals upon intramuscular or subcutaneous injection of endotoxin at high concentrations (Fiske et al., 2001).
Tissue culture is an artificial process for growth, proliferation and maintenance of cells for study and research on the behavior of various animal cells (Durrani et al., 2015, Harison 1907, Freshney 1998).
Cell culture is mainly applied in biological sciences such as virus propagation, vaccine production, diagnosis, hormones production, physiological, biological, pharmaceutical studies, basic , applied researches including cell biology, physiology, pharmacology and toxicology, recombinant protein production, gene therapy, cancer research, drug development, antibodies, interferon, erythropoietin, coagulation factors, safety testing and many other aspects (Durrani et al., 2015, Vester et al., 2010, Park et al., 2010, Marten 2006, Zhang et al., 2013, Balls et al., 1995).
BHK cells are derived from syrian baby hamster kidney (Mesocricetus auratus) (MacPherson and Stoker 1962). BHK cells are inherently anchorage dependent cells but also they are applied as suspension, too (Guo et al., 2015, Hernandez and Brown 2010, Reddy et al., 2016).
BHK cells are mainly used in animal products, particularly for FMD virus vaccine and rabies vaccine production (Vester et al., 2010, Park et al., 2010, Aunin 2010, Kallel et al. 2003, Rahman et al., 2007).
Foot and mouth disease (FMD) is an acute contagious viral disease of cloven footed animals (Orsel et al., 2007). The causative agent is a single stranded positive- sense RNA virus that belongs to the genus Aphthovirus in the family Picornaviridae. There are seven immunologically distinct serotypes of FMD virus, namely; O, A, C, SAT1, SAT2, SAT3 and Asia1 (Belsham, 1993).
In Egypt, The history of FMDV goes back to 1950 (Mousa et al., 1974), when an outbreak caused by serotype SAT2 was reported. Between 1964 and 2005, only serotype O was reported in Egypt (Zahran 1960, and Farag et al., 2005), with the exception of 1972 when type A was introduced from Sub-Saharan Africa (Knowles et al., 2007). Series of outbreaks predominantly caused by serotype O, and with a dramatic upsurge in FMD SAT 2 outbreaks during 2012 were reported (Ahmed et al., 2012, Shawkey et al., 2013). Serotypes O, A and SAT2 have been circulating in the country since 2012, and Serotype O is considered the predominant serotype (FAO 2012).
The control of FMD in animals was considered to be important to effectively contain the disease in endemic areas, so that vaccination of animals is effective in limiting the spread of FMD (Nair and Sen, 1992).
The proper use of good quality vaccines has been a significant factor in the control and / or eradication of FMD (Allende et al., 2003).
The objective of this study was to Screen and control the endotoxin levels during propagation of BHK (Baby Hamster Kidney) tissue culture used in the preparation of FMD (foot and mouth disease) vaccine in VSVRI.
2. Materials And Methods
BHK cell line obtained from (FMD department, VSVRI, Egypt) was cultured in Minimal essential medium supplemented with a set of proteins including lactalbumin, treptose phosphate broth were added into this medium, sodium bicarbonate, and 10% newborn calf serum supplied by Capricorn® with antibiotics penicillin 100 IU/ml and streptomycin 100 IU/ml.
High purity water prepared in VSVRI is used not only for preparation of media and solutions, but also for glassware washing.
Water used for washing all glassware and plastics in FMD vaccine department, VSVRI, is highly purified water prepared by VSVRI, Egypt. Glassware are sterilized in hot air oven at 220°C for 1 hour, while plastics are sterilized by autoclaving at 121°C, 1.5 bar atmospheric pressure for 30 minutes.
The morphological abnormalities of BHK cells were investigated using the inverted microscope.
LAL test was used to screen the endotoxin levels in water, serum, medium with additives, glassware and plastics. This Limulus Amebocyte Lysate (LAL) (Endosafe®) chromogenic assay is very sensitive, detecting down to 0.03 Endotoxin Units (EU)/ml. One EU equals approximately 0.1 to 0.2 ng endotoxin/ml of solution depending on the reference standard used.
Endotoxin Units, rather than units of weight, were developed by the U.S. Food and Drug Administration (FDA) Hoffmann et al., 2005 and Ding 2001.
FMD virus local strains (O /pan Asia2, A/ Iran 05 and SAT2/ Egypt 2012) were locally isolated and were identified by VSVRI, Abbasia, Cairo. These viruses were used to check for the sensitivity of BHK cells.
Polymyxin B Sulfate binds to the lipid A portion of bacterial lipopolysaccharides, disrupting the cytoplasmic membrane by inducing pores large enough to permit nucleotide leakage in bacterial walls. This disrupts the permeability of the cytoplasmic membrane (Xiao et al., 2017). PMB-LPS antagonizing activity occurs at 50 mg/L (Guo et al., 2007).
3. Results and Discussion
Unfortunately, while the sterilization processes will destroy microbial contaminants, endotoxin will be left largely intact. (Roslansky et al., 1991).
Endotoxin elicits a wide variety of pathophysiological effects, such as endotoxin shock, tissue injury, and death (Ogikubo et al., 2004).
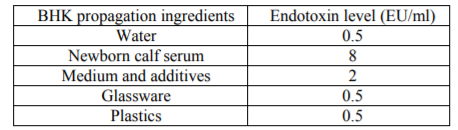
The results of endotoxin screening in the BHK propagation ingredients revealed that highest endotoxin level was 8 EU/ml in newborn calf serum and the least was 0.5 EU/ml in water, glassware and plastics, as shown in table (1).
These results were in agreement with Dumoulin et al. 1991 who mentioned that the endotoxin levels during tissue culture processing should range from 0.5 to 60 EU/ml.
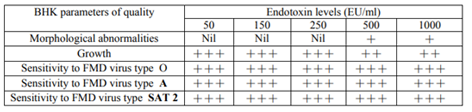
Table (2) shows that only above 500 EU/ml, BHK cells reveal minimal quality alterations. These results were in agreement with Nalbantsoy et al., 2011 who mentioned that BHK cells resist very high levels of endotoxins.
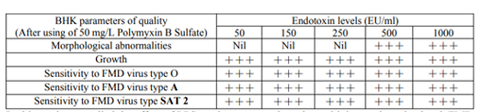
Table (3) shows that Polymyxin B Sulfate neutralizes the endotoxin effect on tissue culture under high endotoxin levels. These results were in agreement with Xiao et al., 2017 who mentioned that Polymyxin B Sulfate binds and neutralizes the lipopolysaccharides.
In this work we studied the effect of LPS on the quality of BHK cells regarding the morphology, growth and sensitivity against the local FMD virus strains in Egypt.
The results revealed that the tissue culture inputs used in VSVRI cope with the permissible LPS levels Dumoulin et al. 1991.
Previous results demonstrated that the BHK cells could withstand high levels of endotoxin burden without showing any alteration from normal (Nalbantsoy et al., 2011).
Results revealed that 50 mg/L of PMB in tissue culture medium could be a magical treatment for LPS contamination Guo et al., 2007, Xiao et al., 2017.
Results supported also by Morrison et al., 1994, Raetz 1990, Raetz 1993, Raetz et al., 1991, they found that endotoxins do not affect all cultured cells equally. Some cell cultures, perhaps lacking appropriate endotoxin receptors, may only be sensitive to very high levels of endotoxin levels not likely to be found in cell culture systems by accident. Cell lines that have been grown in culture for many years (CHO, 3T3, WI-38, BHK, HeLa, etc.) may have been naturally selected for resistance to endotoxin by long term, inadvertent exposure to high levels of endotoxins that could often be found in media, sera and culture additives before endotoxin testing became widely used.
Although BHK cells could resist high levels of LPS, these high levels in parenteral pharmaceuticals could cause a systemic inflammatory reaction, leading to multiple pathophysiological effects, such as endotoxin shock, tissue injury, and death (Anspach 2001, Erridge et al., 2002, Ogikubo et al., 2004).
Finally we recommended the use of pyrogen free tissue culture ingredients and using of polymexin b sulfate as anti-lipopolysaccharides.
References
- Abdel- Rahman, A. O.; Farag, M. A.; Samira El- Kilany; Eman, M. A.; Manal Abo El- Yazed and Zeidan, S. (2006): Isolation and Identification of FMDV during an outbreak of 2006 in Egypt. Kafr El- Sheikh Vet. Med. J.; 4(1): 2006.
- Ahmed, H., Salem, S., Habashi, A., Arafa, A., Aggour, M., Salem, G., Gaber, A., Selem, O., Abdelkader, S. & Knowles, N. (2012): Emergence of Foot‐and‐Mouth Disease Virus SAT 2 in Egypt During 2012. Transboundary and emerging diseases,59:476-481.
- Allende, R., Mendes da Silva, A.J., Darsie, G.C. S., (2003): South American standards for foot and mouth disease vaccine quality. In: Dodet B, Vicari M, editors. Foot-and-mouth disease: control strategies. Paris: Éditions scientifiques et médicales Elsevier SAS; 2003. p. 331–6.
- Anspach,F.B., (2001): Endotoxin removal by affinity sorbents. Journal of Biochemical and Biophysical Methods 49:665-681. 2001.
- Aunin, J.G., (2010): Viral vaccine production in cell culture. In: Flickinger M.C. (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. Wiley, New York, 2010, pp 1–35.
- Balls,M., Goldberg, A.M., Fentem, J.H.,et al., (1995): Report and recommendations of ECVAM Workshop 11. ATIA23, 838, 1995.
- Belsham, G.J. (1993): Distinctive features of FMDV, a member of the Picorna virus family, aspects of virus protein synthesis, protein processing and structure. Progress in Biophysics and Molecular Biology 60: 241-260
- Ding JL, Ho BA. (2001): New era in pyrogen testing. TRENDS in Biotechnology 19:277-281. 2001.
- Dumoulin, J.C.M., Mehheere, P.P.C.A., Evers, J.L.H., Kleukers, A.P.G., Pieters, M.H.E.C., Bras,M. and Geraedts, J.P.M. (1991): The Effects of Endotoxins on Gametes and Preimplantation Embryos Cultured In Vitro. Hum. Reprod. 6:730-734 (1991).
- Durrani, A.,.Mirza, A., Khan, Z., Khan, N., Kulkarni, A., Ali, Y., (2015): Int. J. Appl. Res., 1, 770 (2015)
- Erridge C, Bennett-Guerrero E, Poxton IR. (2002): Structure and function of lipopolysaccharides. Microbes and Infection 4:837-851. 2002.
- FAO (2012): http://www.wrlfmd.org/fmd_ genotyping/africa/egy.htm.
- Fiske JM, Ross A, VanDerMeid RK, McMichael JC, Arumugham. (2001): Method for reducing endotoxin in Moraxella catarrhalis UspA2 protein preparations. J Chrom B 753:269-278. 2001.
- Farag, M.A., Aggour, M. A. and Daoud, A.M. (2005): ELISA as a rapid method for detecting the correlation between the field isolates of FMD and the current used vaccine strain in Egypt. Vet. Med. J. Giza, Vol. 53 no. 4: 949- 955.
- Freshney, R.I., (1998): In: Culture of Animal Cells: A Manual of Basic Technique, Alan R. Liss, Inc., N.Y. 2nd Ed, 1998, pp: 1–3.
- Guo,Y.,B.,1., Chen, L.,P., Cao, H.,W., Wang, N., Zheng, J., Xiao, G.X. (2007): Polymyxin B antagonizing biological activity of lipopolysaccharide. Chin. J. Traumatol. 2007 Jun;10(3):180-3.
- Guo H, Jin Y, Shi-Chong H, Shi-Qi Sun. (2015): Quantitative Proteomic Analysis of BHK 21Cells Infected with Foot-and-Mouth Disease Virus Serotype Asia 1. PLOS ONE, 2015.
- Harison, R.J., (1907): Proc. Soc. Exp. Biol. Med., 4, 140 (1907).
- Hernandez, R., Brown, D.T., (2010): Growth and maintenance of baby hamster kidney (BHK) cells. Curr Protoc Microbiol. Chapter 4: Appendix 4H, 2010.
- Hoffmann S, Peterbauer A, Schindler S, Fennrich S, Poole S, Mistry Y, Montag- Lessing T, Spreitzer I, Loschner B, van Aalderen M, Bos R, Gommer M, Nibbeling R, Werner-Felmayer G, Loitzl P, Jungi T, Brcic M, Brugger P, Frey E, Bowe G, Casado J, Coecke S, de Lange J, Mogster B, Naess LM, Aaberge IS, Wendel A, Hartung T. (2005): International validation of novel pyrogen testes based on human monocytoid cells. Journal of Immunological Methods 298:161-173. 2005.
- Kallel, H., Rourou, S., Majou, S., Loukil, H., (2003): Appl. Microbiol. Biotechnol., 61, 441 (2003).
- Knowles, N. J., Wadsworth, J., Reid, S. M., Swabey, K. G., EL-Kholy, A. A., EL- Rahman, A. O. A., Ssoliman, H. M., Ebert, K., Ferris, N. P. & Hutchings, G. H.(2007): Foot-and-mouth disease virus serotype A in Egypt. Emerging infectious diseases,13:1593.
- MacPherson, I., Stoker, M.,(1962): Virology, 16, 147 (1962).
- Merten, O.W., (2006): Cytotechnology, 50, 1 (2006).
- Mousa,A.A.;Boulaus,S.M.;Elsayed;,F.S.and Bohm,H.O.(1974): Typing and subtyping of a strain of FMD isolated from sharquia province, 1970.J.egypt,assuit Veterinary Medicine, Vol.(34) No.(3-4) : (413-419).
- Morrison DC, Kirikae T, Kirikae F, Lei MG, Chen T. Vukajlovich, S.W. (1994): The receptor(s) for endotoxin on mammalian cells. Prog. Clin. Biol. Res 388:3–15. 1994.
- Morrison, D.C., Dinarello, C.A., Munford, R.S., Natanson, C., Danner, R., Pollack, M., Spitzer,J.J., Ulevitch, R.J., Vogel, S.N., and McSweegan, E. (1994): Current Status of Bacterial Endotoxins. ASM News, 60:479-484 (1994).
- Nair,S.P. and Sen,A.K. (1992): “A comparative study on the immune response of sheep to FMD virus vaccine type Asia1 prepared with different inactivators and adjuvants”. Comp. Immunol. Microbiol. Infect. Dis., 15(2): 117-124.
- Nalbantsoy, A., Karabay-Yavasoglu, N.U., Deliloglu-Gurhan, I., (2011): Determination of in vivo toxicity and in vitro cytotoxicity of lipopolysaccharide isolated from Salmonella Enteritidis and its potential use for production of polyclonal antibody. Food and Agricultural Immunology, 22:3, 271-281.
- Ogikubo Y, Ogikubo Y, Norimatsu M, Noda K, Takahashi J, Inotsume M, Tsuchiya M, Tamura Y. (2004): Evaluation of the bacterial endotoxin test for quantification of endotoxin contamination of porcine vaccines. Biologicals 32:88-93. 2004.
- Orsel, K.; deJong,M.C.; Bouma,A.; Stegeman, J.A. and Dekker,A. (2007): Foot and mouth disease virus transmission among vaccinated pigs after exposure to virus shedding pigs. Vaccine 2 21;25(34):6381-91.
- Park J.H., Park H.H., Park T.H., (2010): Korean J Chem. Eng., 27, 1042 (2010).
- Raetz CR, Ulevitch RJ, Wright SD, Sibley CH, Ding A, Nathan CF. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. The FASEB Journal 5(12):2652-2660. 1991.
- Raetz, C.R.H. (1990): Biochemistry of Endotoxins. Annu. Rev. Biochem. 59:129-170 (1990).
- Raetz, C.R.H. (1993): Bacterial Endotoxins: Extraordinary Lipids that Activate Eucaryotic Signal Transduction. J. Bact. 175:5745-5753 (1993).
- Raetz, C.R., Ulevitch, R.J., Wright, S.D., Sibley, C.H., Ding, A., and Nathan, (1991): Gram-negative Endotoxin: An Extraordinary Lipid with Profound Effects on Eukaryotic Signal Transduction. FASEB J. 5:2652-2660 (1991).
- Rahman, S., Rabbani, M., Sahidullah, Muhammad,K., Iqball,Z.,(2007): Int. J. Agri. Biol., 9, 821 (2007).
- Reddy, B.P., Reddy, B.P., Rayulu, D.J., (2016): Int. J. Apll. Biol. Pharm.Technol., 7, 122 (2016).
- Rietschel, E.T. and Brade, H. (1992): Bacterial Endotoxins. Sci. Amer. 267:54-61 (Aug. 1992).
- Roslansky, P.F., Dawson, M.E. and Novitsky, T.J. Plastics, (1991): Endotoxins and the Limulus Amebocyte Lysate Test. J. Parental Sci. Tech. 45:83-87 (1991).
- Shawky M., Abd El-Aty M., Hiam. M. Fakry, Hind M. Daoud, Ehab El-Sayed I., Wael Mossad G., Sonia A. Rizk, Abu-Elnaga H., Mohamed A. A., Abd El-kreem A. and Farouk E. M. (2013): Isolation and Molecular Characterization of Foot and Mouth Disease SAT2 Virus during Outbreak 2012 in Egypt. J Vet Adv 2013, 3(2): 60-68
- Vester, D., Rapp, E., Kluge, S., Genzel, Y., Reichl, U., (2010): J Proteomics, 73, 1656 (2010).
- Weary, M. and Pearson, F. A Manufacturer’s Guide to Depyrogenation. BioPharm. April, 22- 29 (2007).
- Xiao -Xiao LU, Yi - Fan JIANG, Ying - Ye OU, Zh - De ZHANG, Hong - Ye DI, Dao - Feng CHEN, Yun - Yi ZHANG (2017): Polymyxin B as an inhibitor of lipopolysaccharides contamination of herb crude polysaccharides in mononuclear cells.Chinese Journal of Natural Medicines.Volume 15, Issue 7, July 2017, Pages 487-494.
- Zahran, G.E.D. (1960): Foot and mouth disease in southern region of URA.Bull. Off. Int. Epiz., 13: 390- 393.
- Zhang, X.Z., Y. Chen, H.L. Huang, D.L. Xu, C.B. Ren, B.T. Liu, S. S, Z.X. Tang,. (2013): Pak. Vet. J., 33, 438 (2013).