Information
Journal Policies
Camel Milk Production, Prevalence and Associated Risk Factors of Camel Mastitis Inaysaita Woreda Afar Regional State, North East Ethiopia
Shishay Gramay1*, Mulugeta Ftiwi2
2.Lecturer at Aksum University, Aksum, Ethiopia.
Copyright :© 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The study was conducted with the objectives of assessing the current camel milk production, determining the prevalence, major Bacterial pathogens and associated risk factor of camel mastitis. For this study 100 households and 384 camels were selected randomly. Questionnaire survey was administered to camel holder households to collect data on management practices, knowledge of camel mastitis, treatment attempts and responses, extent of veterinary service delivery, and general information on production and reproduction performances of camels. Clinical examination, CMT and bacteriological culturing were performed to diagnose the occurrence of clinical and subclinical mastitis. Data collected was analyzed using descriptive statistics and univariate logistic regression. GLM (General Linear Model) were used to examine the effect of mastitis, parity, and stage of lactation in camel milk yield. Mean milking frequency and mean lactation length was (3.03±0.731) times a day and (13.8 ± 0.19) months respectively. Diagnosis result of mastitis and stage of lactation had significant effect in milk yield (p < 0.01). However across parity there was no statistically significant difference. Daily mean milk yield was higher (3.6 ± 0.09) in non mastitic camel than the mastitic once (2.3 ± 0.10). The results of CMT and bacteriological culture revealed that Clinical and sub-clinical mastitis were prevalent in 19 (4.9%) and 96 (25%) of the studied animals respectively. This gives an overall CMT based mastitis prevalence of 29.9% (95%CI: 24.93, 34.06) at animal level and 9.4% at quarter level. Additionally, 9 (2.3 %) camels were found with blind teats. The most important pathogens isolated from clinical and subclinical mastitis cases were S. aureus (6.6%), St. dysgalactiae (2.9%), Escherichia coli (2.2%), and S. aureus38 (27.9%), St. Agalactiae16 (11.7%) Escherichia coli (8.1%), Bacillus spp (6.6%) respectively. The univariate logistic regression showed that, among risk factors considered tick infestation (P < 0.01), using ant-suckling device (P< 0.05), stage of lactation (P< 0.01), and parity number (P < 0.05) had significant effect in prevalence of overall mastitis.
Bacterial Pathogen Camelusdromedarius, California Mastitis Test (Cmt), Lactation Length, Milk Yield, Prevalence, Risk Factors.
1. Introduction
The dromedary camel (Camelus dromedarius) is a multipurpose animal adapted to the harsh environments of semiarid and arid zones, essentially kept for milk and meat production and transportation. It is also a financial reserve (asset) and security (drought-prone risk management) for pastoralists and plays an important role in social prestige and wealth. Because of the increasing desertification and recurrence of drought and famine in sub-Saharan Africa, particularly in East Africa, the camel plays a very significant role as a source of milk, meat and draft power (Woubit et al., 2001).
According to Food and Agriculture Organization (FAO) of United Nations, approximately there are 25 million camels in the world where the global market for camel products has a potential of US$10 billion per year. The comparative advantages of the camel as a dairy animal over the other species in the same environment are difficult to quantify; however, in absolute terms, it is widely recognized that the camel produces more milk for a longer period of time than any other animal under the same condition (FAO, 2011).
In Ethiopia camels are kept in the arid and semiarid lowlands of Borena, Ogaden and Afar regions, which cover 50% of the pastoralist areas in the country. The major ethnic groups owning camels in Ethiopia are the Somali, Borena and Afar. Milk of camel is one of the main components of the diet of the nomads in Ethiopia and is consumed in its raw or naturally processed (soured) form (Woubit et al., 2001). According to Behnke (2010)as cited in (Abdi et al., 2011) Ethiopia camel population was estimated to be 2.4 million distributed around the country. In which 458,760 are lactating camels each year with an annual milk production of 608, 315, 760 liters that roughly generates 3, 345, 736, 680 birr (Abdi et al., 2011).
Despite the camel's considerable contribution to food security in semi dry and dry zones and its being a major component of the agro-pastoral systems in vast pastoral areas in Africa and Asia, little is known about its production potential and production systems compared to other domestic animals (Simenew et al., 2013).
Like other dairy animals, dromedary camel could be affected by udder infection as mastitis, a complex disease occurring worldwide among dairy animals, with heavy economic losses largely due to clinical and subclinical mastitis. The last requires indirect means of diagnosis (Matofari et al., 2003). Evidence indicates that subclinical mastitis causes suffering of the animal, reduces milk yield, alters milk properties, impairs preservation and processing and is a public health concern for consumers of camel milk (Tibaryand Anouassi, 2000).
Bacterial infections are considered the primary cause of mastitis in domestic animals. The causative agents of bovine mastitis are well defined but as far as camels are concerned, there is paucity of information about the etiological agents associated with camel mastitis. Very little work is done concerning camel mastitis as the disease was thought to be uncommon in camels (Obeid et al., 1996; Almaw and Molla, 2000; Abdel, 2001). As camels has not been a subject of research, the epidemiology and pathogenesis of these mastitis pathogens remains unclear (Abdurahman, 2006). On the one hand, the disease is not usually treated in traditionally managed camels, hence takes a natural course to chronicity (Obeid et al., 1996). On the other hand, the traditional treatment attempts by herders are usually ineffective. These conditions may lead to chronic, often fibristic sequel resulting in permanent loss of milk production and early culling of the animals (Abera et al., 2010).
Research conducted in camel in any of the disciplines is scant. May be due to the habitat of the camel which is inhospitable for researchers, lack of infrastructure and transportation and to the non-sedentary nature of the herds constantly moving in search for feed and water. In light of this the study was proposed to be undertaken in pastoral area of Ayssaitaworeda of the Afar National Regional State.
- To assess camel milk production
- To determine the prevalence of mastitis in traditionally managed camels
- To identify the major associated risk factors of camel mastitis in the study area
- To identify the major bacterial pathogens cause mastitis in the woreda
2. Materials and Study Methods
Afar region is one of the four major pastoral regions in Ethiopia located in north eastern part of the country. The region is divided in to five administrative zones, which are further subdivided into 29 woredas. The regional population is estimated to be 1.2 million of which 90% are pastoralists and 10% agro-pastoralists. The majority of the land is rocky and the annual precipitation is low (150-500 mm/annum) which makes crop cultivation unsuitable. People in the region, therefore, depend mainly on live stock production for their livelihood. The livestock population in the region is estimated at 703,424 cattle, 1,003,000 heads of sheep, 2,014,418 heads of goats, 301,733 camels and 16,976 donkeys (LCNRDB, 2005).
The study was conducted in AyssaitaWoreda Zone 1 of the Afar Regional State (Figure. 1). Asayita is bordered on the south by Afambo, on the west by Djiubti, on the north by the Awash River which separates it from Elidar, and on the east by Djibouti and 670 km far from Addis Ababa. The woreda consists of 13 Kebeles of which two are urban, five agro-pastora and and six pastoral kebeles. Livestock population of the woreda is estimated to be 115,171 animals; of these, cattle 71,383, goats 23,086, camels 16,943 and 482 equine are found in the area (Aysaita Agricultural office).
A cross-sectional study design was conducted to asses camel milk production as well as prevalence of mastitis, and associated risk factors, and to isolate and characterize the major bacterial pathogens found in the milk of mastitic camels (Camelus dromedarius). Five kebeles were selected purposively from 13 kebeles found in the woreda based on seasonal availability of camels with the assumption of 20 to 30 camels in one herd. Herds in 5 kebeles were taken as sampling frame. The sampling units were lactating camels in a herd. The number of camels that were sampled from a herd was determined proportionally based on the estimation of the camel population. Accordingly 384 lactating camels were selected randomly.
Sample Size Determination for Questionnaire Survey: The sampling units were households keeping camels in the study area. The sample size required for the study was determined by the formula recommended by Arsham (2007) for survey studies as illustrated below:
N=0.25/SE2
Where N= Sample size
SE= Standard Error
Hence, at 5% standard error, the total number of households selected was 100.
Sample Size Determination for Mastitis Prevalence
There is no previous investigation about the prevalence of camel mastitis in the study area. Hence, the average expected prevalence rate was assumed to be 50% for the area within 95 % Confidence Intervals (CI) at ± 5 % desired accuracy. Subsequently, the number of study animals was determined following the formula published in Thrusfield (1995).
n= {1.962 X PexpX (1-Pexp)} / d2
Where
n= required sample size,
d= desired absolute precision,
Pexp= expected prevalence (50%)
Single Visit Formal Survey Methods were applied to collect data. Various techniques and tools such as questionnaires, record sheets and measurements were used to collect information from camel owner households and the camels.
Before milk sample collection questionnaires were administered for camel herders (n=100). Then the record sheets were filled, while taking the milk samples.
The information (questionnaire based) was collected in the villages and settlements before the animals were sent for browsing, or at watering points. The questionnaire and record sheets were pre-tested, and then modified on the basis of the information obtained in the pre-tests. Local ethnic leaders who were known to the respondents were used to interpret and explain the questions in the course of individual interviews and discussions. These leaders were very helpful in communication between the researcher and the respondents.
The questionnaire has mainly focused on management practices, their knowledge of camel mastitis, treatment attempts and responses, extent of veterinary service delivery, and general information on production and reproduction performances of camels. Totally 100 camel owner households were involved in the questionnaire survey based on Arsham (2007) formula.
Whereas the record sheet mainly focuses in risk factors such as production system, age, parity, and milk yield, stage of lactation, tick infestation and type and application of anti-suckling devices were filled simultaneously while taking the milk sample.
Data about parity and lactation stage were gathered by interviewing the owners. The parity number record during the study was parity 1 to 9. The lactation stage 1 to 16 months, lactation stage classifies in to three categories as early (10 days to 2 months), middle (3 to 5 months), and late (>6 months) to observe whether there would be significant difference in the occurrence of mastitis during these stages.
All udders were subjected to clinical examination for presence of swelling, lesions or anatomical malformations prior to sampling; the udder was washed, dried and the teat was disinfected with cotton moistened with 70% alcohol. After discarding the first few squirts of milk about 15 ml were collected in sterile universal bottles and kept in an icebox, and transported immediately to the laboratory for analysis (Samara Animal Health Regional Laboratory) where all the primary biochemical identification tests of the bacteria were accomplished up to genera level. The isolated bacteria were transported to Addis Ababa University College of Veterinary Medicine and Agriculture microbiology laboratory for further analysis (secondary biochemical tests).
The milk samples were examined for their consistency, color and other visible abnormalities. Clinical mastitis was recognized by abnormal milk, signs of udder infection and detection of mastitis pathogens by bacteriological culture; whereas subclinical mastitis was recognized by apparently normal milk and an increase in somatic cells as evidenced by CMT and positive culture results. CMT was used to give an indication of the number of somatic cells present in each of the milk samples.
All collected milk samples were examined for mastitis using California mastitis test. (CMT) was carried out using the method described in Quinn et al. (1994). Equal volumes (2 ml) of commercial CMT reagent (avatar rapid mastitis test Kit-Alvetera Gmbh-Germany) and quarter milk were mixed and the changes in milk fluidity and viscosity were observed. The interpretation of the result was done according to the method described by Quinn et al. (1994). Negative (0) and trace (+/-) were considered as negative and different intensities of positive (1, 2 and 3) were considered as positive (Appendix 2).
The bacteriological culture was performed following the standard microbiological technique (Quinn et al., 1994). One loop full of milk was streaked on 5% sheep blood agar and MacConkey agar to detect bacteria that could grow on this medium. MacConkey plates were used to detect Enterococcus species and any Gram-negative bacteria. Inoculated plates were incubated aerobically at 37°C for 24-48 h. Presumptive identification of bacterial isolates was made based on colonymorphologic features, Gram-stain reaction, hemolytic characteristics, catalase, oxidase and Oxidation fermentation (O- F) tests. Staphylococci and Micrococci were identified based on their growth characteristics on mannitol salt agar, coagulase production, catalase and oxidase tests. Isolates identified tentatively as Streptococci were evaluated according to CAMP reaction, growth characteristics on Edward’s medium (Oxoid), hydrolysis of esculin and sodium hippurate, catalase production, and sugar fermentation tests. Gram-negative isolates were subcultured on MacConkey agar and further tested using triple sugar iron (TSI) agar (Merck), the IMViC test (indole, methyl red, Voges-Proskauerand citrate utilization tests), urea, lysine and ornithine decarboxylase and oxidase reactions.
Data on animal characteristics and herd management practices, including the results of the individual CMT result were recorded and transferred into MS-Excel spread sheet. Data from all questionnaires were verified, rechecked and filtered. Variables with outliers and wide disparities were dropped to prevent bias in the analyses. All the collected data were stored in Microsoft excel spread sheet and transferred to SPSS version 16.0 for analysis.
The CMT screening test data obtained were analyzed to determine the prevalence (by dividing the number of CMT+ lactating camels to the total number of lactating camels tested) and distribution of disease in the lactating -camels of the study area (Thrusfield, 1995). P-value < 0.05 was considered significance.
Significance of risk factors on the prevalence of mastitis in camels were calculated through chi-square (χ2) technique to test the existence of association between CMT positive and risk factors like, parity, stage of lactation, tick infestation and anti-suckling device applied by the herders to prevent the calve from suckling the dam.
Variables those which have significant association as shown from the chi-square (χ2) and P-values with the outcome variable were selected and a logistic regression model was built to illustrate the magnitude of association between the selected factors and mastitis. This was done by delivering odds ratio, p-value and 95% confidence intervals of those selected risk factors when entered as explanatory variables a against the outcome variable (mastitis).
For the purpose of building the logistic regression model, a quarter was defined as CMT positive if it had a CMT score of 1+ or above. A lactating camels was defined as CMT positive if it had at least one quarter with a CMT score of 1+ or above, hence, all CMT scores of negative (−) were coded as 0 and all positive scores of +, ++, +++ were coded as 1. Similarly, all of the explanatory variables were coded appropriately before analysis.
GLM (General Linear Model) were used to examine the effect of mastitis, parity, and stage of lactation in camel milk yield. By computing milk yield as response variable and the diagnosis result presence and/or absence of mastitis, parity and stage of lactation were predictors. The interaction of predictor variables was assessed before computing the GLM there was no interaction effect, therefore the following model was formulated
yijk = µ+ mi + pj + lk + eijk
Where
Yijk = Average daily milk yield (Response Variable)
µ = The overall mean
mi = Diagnosis of mastitis (positive=1, negative=0)
pj = parity (9)
lk = lactation month (3)
eijk = Error term
3. Results
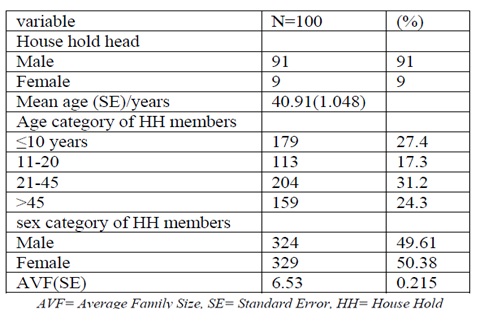
studied households, 91% were male –headed, while the remaining 9% were female headed (Table 1). The average age of the household head was 40.91 years and it range from 24 -70 years. Regarding to the age categories, 31.2% of the house hold members were in the age group between 21-45 years, 24.3% of the house hold members were age above 45 years while 17.3% of the house hold were between 11 – 20 years, whereas the remaining 27.4% of household members were less than 10 years old.
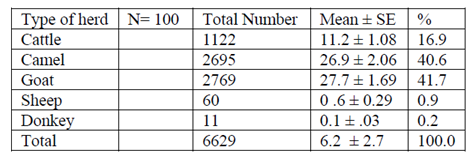
The livestock compositions of the sampled household in Aysaita woreda are presented in (Table 2). From the interviewed household, it was observed that the livestock species composition in the study area was cattle (16.9%), camel (40.6%), goats (41.7%), sheep (0.9%) and donkey (0.16). The mean of camel holding in the study area was 26.95 ± 2.06 heads per house hold.
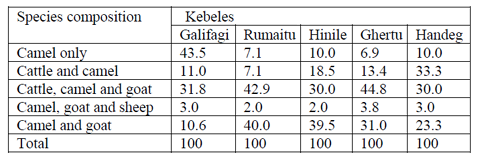
In (Table 3) commonly kept livestock species in the areas were including cattle, goats, sheep, camels and donkeys. Majority of the households were rear cattle, camel and goat simultaneously during wet season, goat and camel during dry season. According to the respondent’s camel is preferred animal during dry season. Commonly kept livestock species in the areas were goats, camels, cattle, sheep, and donkeys in orders of preference. However, Majority of the households were rear Cattle, camel and goat simultaneously.
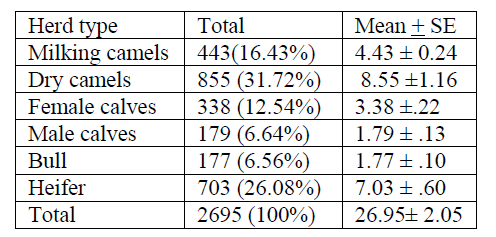
Table 4 indicates total camel category in the herd and the dry camel consist maximum number 855 (31.72%) followed by heifer 703 (26.08%) milking camels 443(16.43%) female calves 338(12.54%), male calves 179 (6.6%) and camel bull the least number 177 (6.5). But the Afar pastoralists mainly depend on milk production. The percentage of lactating camel reported here is unbelievable!!
Hand milking was the only ways of milking camels in the study area the owners prepare the milking vessel (Amure); as part of preparation for milking. During milking period allow the selected calf to come out from the enclosure where calves were kept separately to the open milking area where the dam was kept (not clear statement). Calf was allowed to suckle their dam for a few minutes to stimulate milk let down. Milking the camel was at a standing position with one knee raised to support the milking vessel on his lap. While another man held the calf from suckling. Majority of the respondents (55%) milking were practiced in the morning (7:00 am) and afternoon (2:00-4:00 pm). When the dams goes far from the homestead for grazing during the day time and she back to home at afternoon. The other (45%) respondents milk their camel morning (6:00 am) and on the evening (5:30-6:30 pm).
Milking of camels is practiced in unhygienic environment which is full of dust and dung and without shade in the area. Only male members of the household are responsible for milking camels.
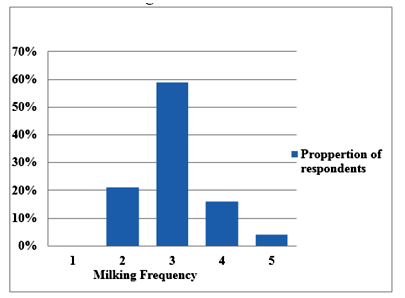
Milking frequency was three times per a day (3.03±0.731). As indicated in (Figure 2) Out of the total sampled camel owners (n=100), 21, 59, 16 and 4 percent of the respondents were indicated that camels are milked twice, thrice, forth and five times a day respectively. (Is that true? Why??)
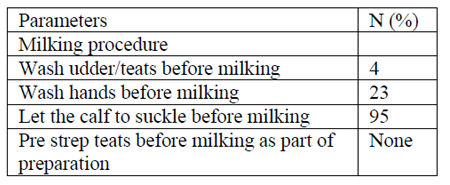
Hygiene before and after milking was unsatisfactory, milking was accomplished in unhygienic environment, washing of the udder and teats of the dams before and after milking were not practiced. About 77% of the respondents have not an experience of washing hand before milking. Only the remaining 23 % respondents have an experience of washing hands when there is availability of water by assuming that clean hands during milking stimulate milk let down.
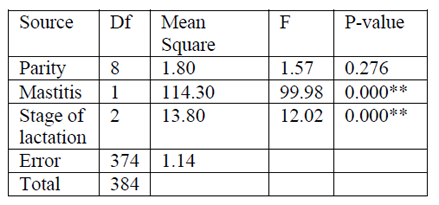
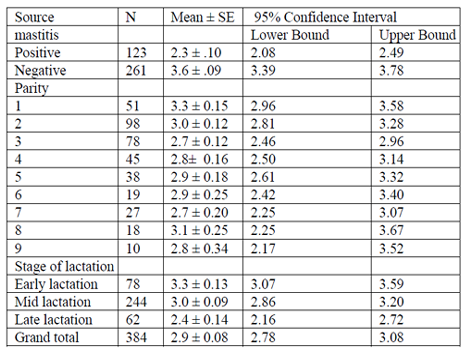
The estimated daily milk yield per camel at different stage of lactation, parity, and diagnosis result (presence and absence of mastitis) were analyzed. The result of univariate analysis of variance is summarized in (Table 7). The estimated mean daily milk yield (± SE) during early, mid and late stages of lactation was 3.3 ± 0.13, 3.0 ± 0.09 and 2.4 ± 0.14 respectively. The average lactation length of camels was between 9-16 months, with the average mean (± SE) of 13.8 ± 0.19 months. Daily mean milk yield was higher (3.6 ± 0.09) in non mastitic camel than the mastitic once (2.3 ± 0.10). Across parity average daily milk yield was no statistically significant association.
Table 6 Indicates diagnosis result of mastitis and stage of lactation had significant difference in milk yield (p < 0.01). However across parity there was no significant difference.
Distance Covered During Grazing
Livestock are congregated around rivers and cotton growing areas in the woreda which utilise dry season grazing areas Joanne et al. (2005). However, it is known that camels are mobile in nature and can cover over a great area for foraging than any other domestic animals. Although the area coverage during grazing depends on the season, availability of feed, adaptability and the knowledge of the area, a distance covered by the camel in searching of feed in the study area were ranges from 2-8 km during wet and extends up to 20 km in dry season. With the mean (±SE) distance of 11.6400 ±.58093.
Migration
According to the information obtained from camel owners, livestock are migrated from Aysaita woreda to neighboring zones in different seasons. During Karma season (July-August) due to the recurrent occurrence of flooding livestock are migrate to Awra, Ewa, Teru, Chifra, Serdo, and Dichiotto. On the other hand, during Sugum season (March-April) livestock do not migrate out from Aysaita due to the abundance of Agricultural by-products such as crop residues which are mainly maize Stover (Hafa), cotton seed and availability of browse species. During the cooler Hagay season (Nov-Jan) pastoralist preferred to return to Aysaita, because during this time flooded water will dry up. Feed condition is better and also agricultural practices are predominantly held in the woreda.
Housing
Camels were housed in open and closed type of houses depending on age. All the respondents indicated that calves camel, and mature camel are housed separately. Mature camels were housed in the open camp around their home. Camel calves were housed in (Gesso) local name of enclosures made for the purpose of keeping calves separately from the rest of the herd. (Gesso) were constructed with wood (Keselto) and fencing by available piece of thorn wood (Prosopisjuliflora) and different bush plants. Thus types of house are constructed with the main objectives of protecting the calves from predators during night time and protect form suckling of dam. The rest of herds’were believed to protect themselves from predators.
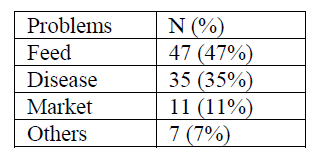
According to the results of interview supported with focus group discussions and field observations held in each of the study kebeles, the major constraint that hinder camel production in the woreda 47%, 35%, 11%, and 7% were listed as feed shortage, diseases, lack of market to sell camel milk products and other problems like the poor genetics of Afar camels for dairy purpose. Difficulty of road, predators, lack of transportation to transport milk from far remote areas to the market, very limited or no access to animal clinics common problems across the woreda.
Feed shortage was further constrained due to encroachment of grazing lands by inedible thorny weeds, (Particularly Prosopis juliflora). Deforestation for the purpose of charcoal making.
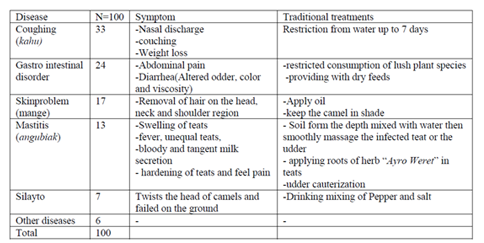
Disease is the second most important factor which hinders the livestock production in general and camel herders particularly in the study area. According to the respondents the type of diseases frequently occurred in the herds in their order of importance were Coughing (33%), Gastro intestinal disorder (24%), Skin problem (17%), Mastitis (angubiak) (13%), Silayto (7%) and other diseases (6%).
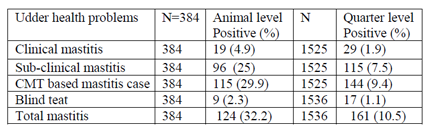
In this study, 384 lactating camels were examined clinically as well as sub-clinically using CMT screening test with subsequent bacteriological examination. Clinical and sub-clinical mastitis were prevalent in 19 (4.9%) and 96 (25%) of the studied animals, respectively (Table 9). This gives an overall CMT base mastitis prevalence of 29.9% (95%CI: 24.93, 34.06) at animal level and 9.4% at quarter level. Additionally, 9 (2.3 %) camels were found with blind teats, and no samples were taken for CMT and culture. Hence, the animal level prevalence (32.2 %) is based on the CMT result excluding the 9 camels with blind teats. Similarly, of the total 1,536 examined teats, 11 (0.7 %) were considered as blind. Thus, milk samples were taken only from 1525 teats, out of which 155 (10.1%) teats were found to be positive for CMT.
Mastitis was prevailed in all study kebeles in different proportion Annex table (4). The higher prevalence was in Handeg (39.7%), Rumaitu (33.3%), Gehertu (31.1%) Galifagi (30.8%) and in hinile were prevailed 25.8% in descending order. However the overall prevalence of mastitis in all studied kebeles were not statically significant difference (P= 0.56, Χ2 = 11.274).
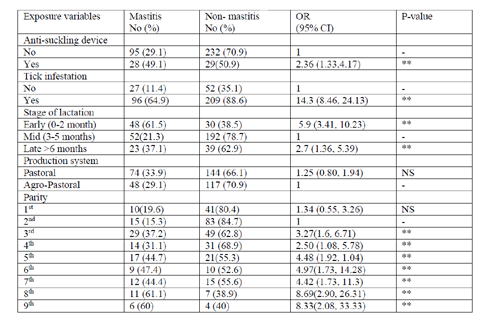
The association of tick infestation, anti-suckling device, stage of lactation, parity and production system with the prevalence of mastitis was showed in Table 11. Accordingly, majority of the tick infested camel udder (64.9%) and those used anti-suckling device (49.1%) were positive for mastitis. Hence, there was a higher likelihood of mastitis occurrence among camels which were tick infested (OR=14.3, 95%CI: 8.46, 24.13; P=0.000) than none infested camels. Likewise, higher likelihood of mastitis occurrence was observed in camels use anti-suckling device (OR=2.3, 95%CI: 1.33, 4.17; P= 0.003) than those not used. At early stage of lactation the occurrence of mastitis were higher likelihood (OR= 5.9, 95%CI: 3.41, 10.23; P=0.000) than mid stage of lactation. Concerning to parity, more or less prevalence of mastitis was in an increasing manner with number of parities. The probability of acquiring mastitis infection was found to be higher in 9th parity (OR= 8.33, 95%CI: 2.08,33.33; P=0.003), in 8th parity (OR=8.69, 95% CI: 2.90, 26.31; P=0.000) than the second parity.
Except in parity two which have a least association and it had been considered as reference to compute the odds ratio. The result of the univariate logistic regression revealed that moreover, parity level had a significant effect on the prevalence of the overall mastitis cases. And no associations were observed between mastitis prevalence and the remaining risk factor (production system).
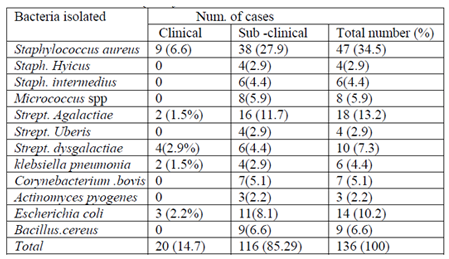
The list, number and proportion of the bacterial isolates from a total of 115 camels and 144 quarters are present in (Table12). Out of the total
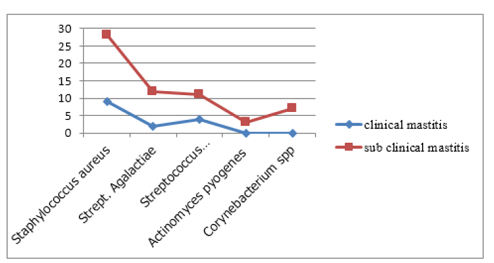
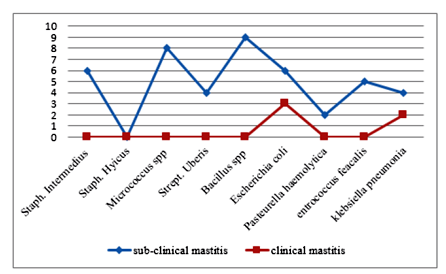
sample cultured 136 (94.4 %) gives bacterial growth, of which 20 (14.7%) were found from milk samples collected from clinically mastitic quarter. The dominant isolates from the clinically mastitic quarter were Staphylococcus aureus, Streptococcus dysgalactiae, Escherichia coli, and Streptococcus agalactiae. While 85.3% (n=116) of isolates were found from milk samples originating from camels with sub-clinical mastitis, Staphylococcus aureus, Streptococcus dysgalactiae, Streptococcus. agalactiae, Staphylococcus hyicus, Escherichia coli and B.cereus were commonly isolated bacterial species.
Staphyl ococcus aureus was the most frequently recorded bacterial species accounting for (34.5 %) of all isolates, followed by Streptococcus Agalactiae (13.2), Streptococcus dysgalactiae (9.5), Staphylococcus hyicus (2.9%), Bacillus species (6.6%), Escherichia coli (10.2), Micrococcus spp (5.9),Coryne bacterial species (5.1%). Staphylococcus intermidius (4.4%),klebsiella pneumonia (4.4), Strept. Uberis (2.9%), and Actinomycespyogenes accounted for (2.2%) of the isolates. Based on their origin 62.3%of the isolates from both clinical and subclinical cases were contagious pathogens (Figure3). While 37.7 % were environmental pathogens (Figure4).
4. Discussion
As in most of other pastoral societies, livestock herding is the main activity of the pastoralists in the study area. The pastoralists in the area possess mixed livestock species, the composition of which varies depending on the availability of grazing pasture, browsing plants and climatic condition of the area. In terms of number, the predominant livestock species kept by the pastoralists was the goats (41.7%) followed by camel (40.6%), cattle (11%), sheep (0.6%) and donkey (0.1%) accordingly. The present result reveals to the findings of Simenew et al. (2013) who reported that Camel, goat, cattle, sheep and donkey are livestock species in the ranking order of their relative preference. In Afar pastoral area as is true in most of other pastoral systems camel is the best adapted animal because of its ability to resist drought and water deprivation because of their special physiological and anatomical adaptation mechanisms over other domestic livestock species.
The proportions of livestock type vary with the availability of grazing pasture and browsing species of plants. The pastoral kebele Galifagi, which were located around Aysaita Town near to the stream of Awash River and to the newly established sugar factory, was hold large number of cattle, camel and goats than the other kebeles. Whereas in Gehirtu Camels were the major livestock type found in the area since the area was dominated by bush and browsing shrubs. More over pastoralist were kept mixed herds in the study area. This result is similar to (Ramet, 1989) who noted that In Somalia pastoralists keep mixed herds with varying milking capabilities, thus assuring a continuous supply of milk throughout the year.
The pastoralists have an endogenous knowledge of risk minimization strategies which helps them to maintain their pastoral ways of life. Herd maximization and diversification are the most appropriate strategies in Afar pastoralist communities. Herd maximization provided a means for efficient utilization of available range resources. Most of the time in pastoral area there is spatiality of rainfalls as a result availability of vegetations is also highly variable from place to place. During such conditions herd maximization and diversification be coming in place to utilize the available resources.
This result is also consistent to the reported by (Kelemework, 2001) present as the different species have different feeding habits, severe degradation was avoided by maintaining a relatively low grazing density. Diversification was also economically advantageous because the different species were utilized for different purposes (Kelemework, 2001). An Afar pastoralist prefers not to sell camel, though it fetches much more market value than cattle and goats. Camels are sold only during times of crisis. Most of the time, they sold male camels. Gift of camel is a sign of high honor and is used to cement relationships between mutually dependent groups and individuals.
The mean camel herd size in the study area was 26.9 ± 2.06. This finding is incompatible with the previous finding of Bekelle (2010) who reported 18.4±1.8 in Borana plateau. However, this result is close to 25.2± 2.2 camels for Gabra pastoralist reported by the same author. This figure indicates how important camels to the pastoralists in the area.
From the total camel category in the herd, the dry camel consist maximum number 855 (31.7%) followed by heifer 703 (26.0%), milking camels 443 (16.4%), female calves 338 (12.5%), male calves 179 (6.6%) and camel bull the least number 177 (6.5%). Among the total number of camels owned by a given household, the number of female camels far exceeds that of male camels. The preference for more females than males is to ensure good supply of milk and reproductive potential.
The statement is also proved by majority of respondents’ that milk production was major objectives of keeping camel and the strong interest to increase camel herd size and this is in agreement to reports of Yayneshet and Kelemework, (2004), Afar pastoralists have the tendency of maximize females while keeping males at the minimum number in herd structure to ensure effective breeding and food security through market exchange of livestock.
This result is also consistent with Megersa et al. (2008), and Getahun and Kassa (2002) in Ethiopia who reported most pastoral herds have higher proportion of breeding female 50%, and 51% respectively. Male camels are mainly used as a pack animal in the area. For the transport of people, goods and mobile houses of the pastoralists during their seasonal migration in search of feed and water for their animals. Some respondents also rent their male camels for salt traders for transportation purpose.
Feed shortage, disease prevalence, lack of market and animal clinic were the major problems which hinders livestock production in order of importance in the study area.
This result reveals to the findings of (Alemayehu, 2001) who reported that, the major problems of camel production in (Afar and Kereyu) include disease, shortage of feed water, marketing problems and poor genetic potential of camels and it is also similar with the result reported by Ahmed (2002) noted that the major milk production constraint is lack of road infrastructure to transport milk from far remote areas to the market.
Hand milking was the only ways of milking camels in the study area. Before milking the calf was allowed to suckle its dam for a few minutes to stimulate milk ejection. After this, one man holds (protects) the calf while another man milks the camel at a standing position with one knee raised to support the milking vessel on his lap. Milking is practiced morning, afternoon, and evening, two to five times a day with the average mean of 3.03±0.731. This finding is consistent with Ahmed (2002) who stated that the gel-lab camel was milked on average 3.61 times a day.
Milking was practiced in an area which is fully exposed to environmental micro organisms which may contaminate milk. And only male members of the household are responsible for milking camels. This observation was strongly agreed with the findings of Eyasu (2009) who reported unlike the case in the highlands of Ethiopia where women are responsible for milking of dairy cows, in Borana usually male members of the household milk camels.
Sometimes the man washes his hand by mixing of soil and water in order to clean his hand before milking. Washing of the udder and teats of the dams before milking was not practiced. About 77% of the respondents have not an experience of washing hand before milking. Only the remaining 23 % respondents have an experience of washing hands when there is availability of water by assuming that clean hands stimulate milk let down. This result is in disagreement with findings of Eyassu (2009) who reported that; 39% of respondents from Borana were the experience of washing hands before milking. This difference could be due to; difference in awareness of herders about udder health problems and difference in availability and distribution of water.
The average daily milk yield per camel observed in this study (3.43 ± 0.18) is fairly in agreement with daily milk yield of Afar camel ranges from 2.01 to 12 liters per camel reported by Simenew et al. (2013). But the result is incompatible with the results reported by (Knoess, 1977) and Gebre-Mariarm (1987) who reported in Ethiopia camels produced about 8 liters of milk a day when milked twice a day, totaling 2,470 kg in 305 days and average daily milk yield of Somali camels ranged between 5 and 6 litres respectively. In this study, the estimated mean daily milk yield in early, mid and late stage of lactation were 4.13, 3.41 and 2.65 respectively which is much more lower than the findings of Yohanse et al. (2007) who reported that estimated mean daily milk yield during early, mid and late stages of lactation in Babilie and Kebribeyah was 6.41 and 5.01, 5.00 and 5.12 and 3.20 and 3.19 liters respectively. Similarly, the result of this study is lower than the previous finding reported by (Basmaeil and Bakkar, 1987) the average milk yield was 5.5 ± 1.5 litres.
The average lactation length of camels observed in this study (13.8 ± 0.19 months) was slightly higher than the values reported by Eyassu (2009) (382.7 days), Bekele et al. (2002) (353 ± 14 days) and Kebebew and Baars (1998) (282 days) and much higher than Alemayehu (2001), which is 6 to 8 months for Afar and Kereyu camels of Ethiopia respectively. But in agreement with findings of Tezera (1998) who indicated 13 and 15 months for Shinile and Jijiga camels, respectively. The lactation length recorded in the present study is, however, shorter than the findings of Schwartz and Walsh (1992), who indicated it to be 15 to 18 months. This variation may originate from breed/type, agro-ecological and seasonal differences.
In general the great variation in camel milk production may be emanated from methods employed to determine yield, high genetic variation between individuals, breed, feeding and management conditions, type of work, milking frequency, and persistency of lactation, and stage of lactation.
Concerning to animal feed resource and camel housing in the woreda, the current observation were similar to Joanne et al. (2005) who reported that livestock feed comprises grazing and browsing of communally owned rain fed rangelands in Afar. The current result is also in concordance to the findings of Eyassu (2009) who noted that camels in Shinile and Jijiga zones, eastern Ethiopia were fed exclusively on natural browse; herded during the daytime on communal grazing lands and kept at night in traditional enclosures (Corral) made of thorny bushes and tree branches as protection from predators. This is similar to the Afar (Gasso) an enclosure where calves were kept separately from the herds, to protect suckling and to safe guard during night.
From the field observation and questionnaire results the attitude of camel herders towards disease identification and treatment were good (they have endogenous knowledge in disease identification and treatments). Concerning to mastitis majority of the respondents (85%) was aware of mastitis as disease locally called “angubiak” for clinical mastitis which is higher than the findings reported by Alemayehu (2013) only 70.1% of respondents had awareness about camel mastitis in Borana. This could be due to the repeated exposure of occurrence of the disease in the study area. However 94% of respondents were not conscious of subclinical mastitis and the way of transmission and control mechanism of the disease. This result disagree with fidings of Alemayehu et al., (2013) who reported 57 % of respondents from Borana were do not have the awareness about the way of transmission of disease and its control This could be due to the nature of the disease which is not diagnosis directly through naked eye, loss were occurred without the knowledge of the farmers. As a result most of the herders were believed that a camel does not affected by udder disease. Rather they give more attention to diseases like coughing and gastro intestinal problems that shows clinical signs.
Therefore, the traditional hand milking and attitude of farmers towards mastitis contributes to the dissemination of mastitis and consequently milk loss in the study area. The major traditional treatments of camel mastitis (angubiak) in the area were deep soil from the surface and mix with water then smoothly massage the infected part and also use roots of herb “AyroWeret” to apply in the infected teat or udder. Cauterization of udder is also common practice in case of clinical mastitis.
The overall prevalence of mastitis (32.50%) and 10.1% clinical mastitis are in agreement with Bekele (2010) who reported the overall mastitis at animal level in Borana, lowland of Ethiopia ranges from 28.6% to 37% and the clinical mastitis 10% respectively. But the overall prevalence of mastitis reported by Bekele and Molla (2001), Fasil et al. (2010), Eyassu, et al. (2010), Alemayehu et al. (2013) were 59.8%, 66%, 75.5, and 44.8 respectively which are greater than the findings of present study. Almaw and Molla (2000), and Abera et al. (2010) reported (2.1%) and 29% respectively of mastitis prevalence in lactating camels in north eastern Ethiopia. Prevalence rate of 4.9% for clinical mastitis, in this study was lower than the prevalences of 19.5%, 12.5%, and 8.3%, reported by Obeid et al. (1996) Bekele and Molla (2001), and Abera et al. (2010), respectively. On the other hand, lower clinical mastitis of 2.1% was reported by Woubit et al. (2001). According to Alemayehu et al (2013) the CMT-based quarter level prevalence of camel mastitis in Borana Zone, Oromia Regional State, Ethiopia was 22.3 % which is higher than the present prevalence.
The overall prevalence of camel mastitis observed at camel and quarter level was low compared to the results of previous studies in other parts of the country. This could be due to difference in breed used, management system employed and Agro-ecology Chafe et al. (2008). Occurrence of mastitis may be influenced by some heritable characteristics such as capacity of milk production, teat structure and udder conformation as well as genetic variation in disease resistance among breeds (Abdurahman, 1995).
In traditional husbandry practices of the study area, anti-suckling device, fibers from plants or strip of cloth are tied to the teat to prevent the calf from suckling the dam. The present finding is consistent with the findings of Almaw and Molla (2000) who reported that camel herders in the Afar tie the teats with soft bark to prevent the calf from suckling when calves began to herd together with their dams. It is also observed that they apply cattle dung in to udders of lactating camels and put sticks in to the nostrils of calves to prevent suckling.
The udder is a predilection site for tick infestation which causes skin and teat lesions, facilitates bacterial entry and leaves behind permanent tissue damage (Woubit et al. 2001). In the present study the incidence of mastitis was higher in tick infested (64.9%) than non infested (11.4%) udders. This result is consistent with Obeid et al. (1996) who indicated that heavy tick infestation of the udder, harmful treatment of affected quarters by cauterization and use of anti-suckling device could be some of the factors, which predispose camel udders to bacterial infection. Similarly, Woubit et al. (2001) reported high prevalence of mastitis in dromedary camels in Borena. The apparently high prevalence of mastitis in the camel herds examined might be attributed to the high tick infestation rate.
The result of univariate analysis revealed that the parity level had a significant effect on the prevalence of the overall mastitis cases. This result is consistent with the findings of Abera et al. (2010) and Riyadh et al. (2011) who reported age and parity number affected mastitis prevalence, there was an increasing trend of mastitis prevalence with increasing parity number and the risk of subclinical mastitis increased significantly with parity and the early stage of lactation. The possible reasons could be as the age (parity) of the camels increase physiological defense mechanisms of the udder or the milk reduce, so that minor pathogens and opportunistic organisms get access to the glandular tissue and cause inflammation of mammary gland (Abera et al., 2010).
Moreover the treatments for the cure of the disease may not be effective, as a result the camels may persistently infected or diseases may persist up to next parity. The other possibility is that as the time of exposure to wards different risk factors increase the probability of having an infection is also increase.
The first stage of lactation could be associated with decreased resistance of mammary gland to infection as result of immune depression following the stresses and hormonal changes that occur around the time of parturition and onset of lactation may leads to high prevalence of subclinical mastitis (Sordillo, 2005; Burvenich et al., 2007).
Production system does not have significant effect in prevalence of mastitis in present study.
This may be due to the similarity of camel husbandry practices. The management of the camel owner households was largely similar.
Gram positive cocci were the main bacterial isolate in mastitis cases. S. aureus, was identified as the most common bacteria. The organism constitutes 47 (34.5%) of the total isolates. This finding is in line with that reported by Karmy (1990), Obeid et al. (1996), and Fasil et al. (2010), who reported (34.4%), (31.5%) and (28%) respectively. The result also agrees with the report of Abdurahman (1995) that CNS and S. aureus represented 61.1% and 38.9%, respectively of the total isolates and considered as the main organisms that cause mastitis in the Bactrian camel.
The high prevalence of S. aureus may be attributed to wide distribution of the organisms inside the mammary gland and the skin of teats and udder and its frequent colonization teats (McDonald, 1997). From the overall mastitis cases Streptococcus constitutes (25.4%) of which St. dysgalactiae and St. Agalactiae were prevailed 13.2 % and 9.5% isolates respectively. This finding agrees with that reported previously (Abdurahman, 1995; Woubit Salah et al., 2001). St. agalactiae and S. aureus were reported to be the most common causes of camel mastitis in eastern Sudan (Obied et al., 1996) and Kenya (Younan et al., 2001).
The commonly isolated genera of bacteria Staphylococcus, Streptococcus, Coryne bacterium, Bacillus, and Escherichia in this study agree with Sibtain et al. (2011), Bekele and Molla (2001), Sena et al. (2001), Younan et al. (2001), Matofari et al. (2005), Abdurrahman isolates. This result is consistent with findings of Fasilet al. (2010) who reported that, E.coli (2006), Kalla et al.(2008), and Abera et al. (2010) who found Staphylococcus, Streptococcus, and Escherichia as major mastitis causing pathogens.
The results of investigations carried out by Obied et al. (1996), Almaw & Molla (2000), Sena et al. (2000) and Abdel Gadir et al. (2005) showed that coagulase positive (CPS) and negative staphylococci (CNS) are the bacteria most frequently isolated from camels and can be considered as main reason for subclinical mastitis in dromedaries. represents 11.8% of isolates in mastitic camel milk.
Based on their origin 60.1%of the isolates from both clinical and subclinical cases were contagious pathogens. While 39.1 % were environmental pathogens. This could be due to unhygienic milking practice in the study area.
5. Conclusions And Recommendations
Feed shortage and disease prevalence were pre dominant factors which hinder livestock production and productivity. The livestock species which are commonly kept in the study area were goat, camel, cattle, sheep and donkey in descending order. Among them, camels were mainly kept for milk production and reproduction. It produces valuable amount of milk for long lactation length under harsh environment. This study reveals moderate to high prevalence of mastitis at camel and quarter level in the study area and mastitic camel was prone to daily milk yield reduction than the non mastitic once. The potential risk factors like tick infestation, anti-suckling device, parity and stage of lactation were highly associated with the overall mastitis cases. Gram positive cocci were the main bacterial isolates in mastitis cases. Staphylococcus and Streptococcus species were the dominant bacterial isolates in the study area. Based on the above conclusion the following points are forwarded as recommendations
- Integration of livestock feeding with crop production and conventional feeds like Molasses Urea Block (MUB) from the recently established Sugar Factory (using by products as source of livestock feed) minimize feed shortage problems
- As in other pastoral parts of Ethiopia camel herders have no access to animal clinic in the study area. Thereforeit is further advocated that mobile animal clinic is necessary to improve udder health and camel milk production.
- To change and improve the livelihood of the pastoral and agro -pastoral communities by improving camel milk production, awareness creation on camel mastitis (particularly sub clinical mastitis) for herders, animal health workers and clan leaders through consistent training which centered on the indigenous knowledge should be practiced continuously.
- In order to reduce the high prevalence of contagious and environmental mastitis in the area, following mastitis control program, improved milking hygiene, prevention of skin lesions, culling of chronic mastitis carriers, and treating of clinically infected she-camels should be practiced.
- Further research should be done to investigate the performance of Afar camel as dairy animal, economic loss due to mastitis at animal level, moreover, investigation on mastitis causing pathogens at molecular level and their association with potential risk factors is necessary
References
- Abbas B, Al-Qarawi A, Al-Hawas A (2000). Survey on camel husbandry in Qassimregion, Saudi Arabia: Herding strategies, productivity and mortality. Revue D'Livest.Vet. Med. Trop., 53: 293-299.
- Abdel G, Hildebrandt G, Kleer J, Molla B, Kyule M, Baumann M (2005). Prevalence and risk factors of camel (Camelus dromedarius) mastitis based on bacteriological examinations in selected regions of Ethiopia. J. Camel Pract. Res., 12:33 – 36.
- Abdi A, SeidM, Abdurehman E (2011). Town camels: pastoral innovation in a fast changing world. A case study from GodeTwon,Somali Regional State, Ethiopia. Paper presented at the international conference on the future of pastoralism 21-23 march.
- Abdurahman O (2006). Udder health and milk quality among camels in the Errer valley of eastern Ethiopia.Lives.Res. Rural.Dev., Vol. 18No. 8. Web site
- Abdurahman O, Agab H, Abbas B, Astorm G (1995). Relation between udder infection and somatic cells in camel (Camelus dromedarius) milk.Acta. Vet. Scand., 36:424-431.
- Abera M, Abdi O, Abunna F, Megersa B (2010). Udder health problems and major bacterial causes of camel mastitis in Jijiga, Eastern Ethiopia: implication for impacting food security, Trop. Anim. Hlth. Prod., 42: 341–347.
- Ahmad S, Yaqoob M, Hashmi N, Ahmad S, Zaman M, Tariq M (2010). Economic importance of Camel: a unique alternative under crisis. Department Of Livestock Management, University Of Agriculture, Faisalabad. Type publication?
- Ahmed M (2002). Study on practices and problems of camel production in Afder zone of Somali national regional state, Ethiopia. MSc Thesis Alemaya University 148p.
- Al-ani F, Al-shareefi M (1997). Studies on mastitis in lactating one humped camels (Camelusdromedarius) in Iraq. J. Camel Pract. Res., 4(1), 47 – 49.
- Alemayehu G (2001). Breeding program and evaluation of semen characteristics of camels in the central Rift Valley of Ethiopia. MSc Thesis Presented to the School of Graduate Studies of Alemaya University 67p.
- Alemayehu R, Gelma G, Dawit T, Fufa A, Bekele M (2013). Prevalence, risk factors, and major bacterial causes of camel mastitis in Borana Zone, Oromia Regional State, Ethiopia.doi.10.1007/s11250-013-0403-6. Springer Science+Business Media Dordrecht 2013.
- Al-Majali A, Al-Qudah K, Al-Tarazi Y, Al-Rawashdeh O (2008). Risk factors associated with camel brucellosis in Jordan. Trop. Anim. Hlth Prod., 40: 193-200.
- Almaw G, Molla B (2000). Prevalence and etiology of mastitis in camels (Camelusdromedarius) in eastern Ethiopia.J. Camel. Pract. Res., 7: 97-100
- AL-Tofaily Y, Al Rodhan M. (2011). Study on Clinical Mastitis (Bacteriological) in She-Camels (Camelusdromedarius) in Some Areas of Middle Euphrates in Iraq.AL-QadisiyaJ. Vet.Med.Sci. 10 (2).
- Antonio R (2009). Livestock’s and pastoralists. Senior Technical Adviser on Livestock and Farming Systems Technical Advisory Division.
- Arsham H (2007). Perturbed Matrix Inversion with Application to Linear Programs SimplexMethod. Applied Mathematics and Computation, 188: 801-807.
- Barbour E, Nabbut N, Frerichs W, Al-Nakhli H, Al-Mukayel A (1985). Mastitis in Camelus dromedarius in Saudi Arabia. Trop. Anim. Hlth. Prod., 17: 173-179.
- Barker A, Schrick F, Lewis M, Dowlen H, Oliver S (1998). Influence of clinical mastitis during early lactation on reproductive performance of Jersey cows. J. Dairy Sci., 81: 1285–1290.
- Basmaeil S, Bakkar M (1987). Milk production of Majaheem camels in Saudi Arabia during the first lactation season. In Proc. Saudi. Biol. Soc. Jeddah, 10:75-79.
- Becker B, Becker H, Bürk C, Dietrich R, Märtlbauer E (2005). Bacillus cereus. Behr's Verlag, Hamburg.
- Behnek R (2010). The Contribution of Livestock to GDP in the IGAD Member States: Study Findings, Applications of the Methodology in Ethiopia and Recommendations for Extension of the Programme. IGAD LPI (IGAD Livestock Policy Initative) working Paper No. 02-1, Addis Ababa, Ethiopia.
- Bekele M (2010). An epidemiological study of major camel diseases in the Borana lowland, Southern Ethiopia. DCG (Dry lands Coordination Group) Report No. 58.
- Bekele T,Molla B (2001).mastitis in lactating camels (Camelus dromedarius) in afar region, north-eastern Ethiopia. Berl Munch Tierarztl Wochenschr; 114 (5-6):169-72.
- Benkerroum N, Boughdadi A, Bennani N, Hidane K (2003). Microbiological quality assessment of Moroccan camel’s milk and identification of predominating lactic acid bacteria. Available online at http://www. academicjournals.org.
- Bernard F (2008). The production potential and importance of camels, camelids in the world. CIRAD-ES, Campus International de Baillarguet, 34398 Montpellier, France. Can inform present animal genetic resources policy development.Anim. Genet. Res. Inf., 41:1-4.
- Bhatt L, Chahar F, Tuteja R, Tanwar K, Verma D (2004). Therapeutic efficacy of enrofloxacin alone and in combination with levamisole in Subclinical mastitis in camel.J. Camel Pract. Res., 11: 153 – 156.
- Blood D, Radostits O (1989). Veterinary Medicine: A text book of the disease of cattle, sheep, pigs, goats and horses.7thedn. Bailliertindall, London. 501-550.
- Bradley A, and Green M J(2001). Adaptation of Escherichia coli to the bovine mammary gland.J. Clin. Microbiol.,39: 1845 – 1849.
- Bramley A J, and Dodd F (1984). Mastitis control: progress and prospects. J. Dairy Sci., 51: 481.
- Burvenich C, Bannerma DD, Lippolis J, Peelman D L, Nonnecke B J, Kehrli J, Paape M J (2007). Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J. Dairy Sc., 90: 39-54.
- Capuco A V, Bright S A,PankeyJ W, WoodD L, Miller R H, Bitman J (1992).Increased susceptibility to intramammary infection following removal of teat canal keratin.J. Dairy Sci., 75: 2126.
- Carter G R, and chengappa M M (1991). Essentials of veterinary bacteriology and mycology.4th Edition, Lea and Febiger, Philadelphia, 109- 243.
- Carter J R (1984). Diagnostic procedure in veterinary bacteriology and mycology. 4th ed. USA: Charles C. Thomas publisher. Pp 367-395.
- Chaffer M, Leitner G, Glickmann A, Van creveld C, Winkler M, Saran A, Yagil R (2000). Determination of udder health in camels.J. Camel Pract. Res., 7: 171 – 174.
- CSA (2009). agricultural sample surve volume II report on livestock and livestock characteristics. Federal Democratic Republic of Ethiopia Central Statistical Agency (CSA).
- De Graves F J, Fetrow F (1993). Economics of mastitis and mastitis control. The Veterinary Clinics of North America: Update on Bovine Mastitis, Ameri J. Sci. 9: 421–434.
- DohooI R, Martin S W (1984). Disease, production and culling Holstein Friesian cows. Pre. Vet. Med., 2: 671-668.
- Eberlein V (2007): Hygienic status of camel milk in Dubai (United Arab Emirates) under two different milking management systems. Thesis for the attainment of the title of Doctor in Veterinary Medicine from the Veterinary Faculty Ludwig- Maximilians- Universität München.
- EFSA (2003). Opinion of the scientific panel on biological hazards on a request from the commission related on tuberculosis in bovine animals: risks for human health and control strategies. European Food Safety Authority (EFSA), EFSA J., 13: 1 – 52.
- El-jakee J (1998). Microbiological studies on mammary glands of one humped she-camels in Egypt. J. Camel Pract. Res., 5: 243 – 246.
- Erb H N, Smith R D,Hilman R B (1988). Rates of the diagnosis of six diseases of Holstein Friesian cows during 15 day and 21 day interval. Am. J. Vet. Res., 45: 333-335.
- Erskine R J (2001). Mastitis control in dairy farms. In: Radostits, O.M. (eds.), Herd health, Food Animal Production Medicine. 3rdEdition, W.B. Saunders Company, Philadelphia, Pennsylvania, Pp 397-432.
- Erskine R J, Ebrehat R J, Hutchinson S B (1988). Incidence and type of clinical mastitis in dairy herds with high and low somatic cell count. Am. J. Vet. Med. Assoc., 192: 761.
- EyassuS (2009). Analysis on the contributions of and constraints to camel production in Shinile and Jijiga zones, eastern Ethiopia.J. Agri and Envir. Int Devel., 103 (3): 213-224.
- Eyassu S, Bekele T (2010). Prevalence and etiology of mastitis in traditionally managed camels (Camelusdromedarius) in selected pastoral areas in eastern Ethiopia.
- Fasil M, Baylegne M, Abrahma A (2010). Camel mastitis associated Bacterial pathogens and its impact in on milk quality in gewane district, afar regional state, north eastern Ethiopia. Bull. Anim. Hlth. Prod. Afr., 58: 249-259.
- Faye B (2004). Dairy productivity of camels. proc. Of the 34th meeting FAO/ICAR (International committee for animal recording). Session on camelids. Sousse (Tunisie).
- Food and Agriculture Organization (FAO) (2011). World Livestock 2011.Livestock in food security. Rome, FAO. Available online at http://faostat.fao.org/site.
- Gebre-Mariam A (1987). Livestock production and its socio-economic importance among Afar camels of northeast Ethiopia. Camel Forum Working Paper No.16.Mogadishu and Uppsala.
- Getahun T, KassaB (2002). Camel Husbandry Practices in Eastern Ethiopia: The Case of Jijiga and Shinile Zones. Nomadic Peoples 6, 158.
- Glipha (Global Livestock Production and Health Atlas) (2006). Global livestock production and health atlas of the FAO. Available online at http://www.fao.org/ag/aga/glipha/index.jsp.
- Grommers F J (1988). Host resistant mechanism of the bovine mammary gland: An analysis and discussion. Neth. Milk Dairy J., 42: 43.
- HabbitK G, Cole C B, Reiter B (1969). Antimicrobial proteins isolated from the teat canal of the cow. J. Gen. Microbiol., 56: 365.
- Haddad G (2006). ‘Camel Milk and Urine Hadiths’, Available online at http://mac.abc.se/ ~onesr/f/.
- Hafez A M, Razig S A, El-amrousi S, Ramadan R O (1987). Studies on mastitis in farm animals in Al-Hasa; Analytical studies. Assiut Vet Med. J., 19, 140 – 145.
- HjortAfOrnas (1988). Sustainable subsistence in arid lands: the case of camel rearing. Available online at http://a-z-animals.com/animals/camel/
- IbnoafM (1987). Towards a better understanding of our domestic livestock: Camels of Kuwait (Technical Report-Field Survey). Kuwait Institute for Scientific Research, Safat, Kuwait.
- Ibrahim A (1990). Potentialities for milk production from Sudanese she-camels in feedlot system. In proceeding of international conferences in Camel production and improvement, 10-13 December, Tobruk, Libya.
- Ilona G (2009). Elmt “Good practice” bibliography. Camel health and husbandry. By Ilona Gluecks, Vétérinaires Sans Frontières (VSF) Suisse. Publication type
- Ismail M D, AI-Mutairi S E (1990). Production parameters of Saudi camels under improved management system. In proceeding of international conferences in Camel production and improvement, 10-13 December, Tobruk, Libya.
- Joanne P, Asnake A, Kassayen H (2005). livelihoods/emergency assessment in Afar region, Oxfam International.
- Jones G M (2006). Understanding the basics of mastitis. Virginia Cooperative Extension. Publication No. 404-233. Virginia State University, USA, pp: 1-7.
- Kalla D J U, Butswat I S R, Mbap S T, Abdussamad A M, Ahmed M S, Okonkwo I (2008). Microbiological examination of camel (Camelus dromedarius) milk and sensitivity of milk microflora to commonly available antibiotics in Kano, Nigeria, Savan. J. Agri, 3: 1–8.
- Kamoun M, Ellouze S, Grondin l, QuiziniCh (1990). The Tunisian experience in camel milk production and processing. In proceeding of international conferences in Camel production and improvement, 10-13 December, Tobruk, Libya.
- Kaper J B, Nataro J P, Mobley H L T (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol., 2: 123 – 140.
- Kapur M P, KhannaB M, Singh R P (1982). A peracute case of mastitis in a she-camel associated with Klebsiella pneumoniae and Escherichia coli. Indian Vet. J., 59: 650 – 651.
- Karmy S A (1990). Bacteriological studies on mastitis in small ruminants and she-camels in Upper Egypt. J. Egypt Vet. Med. Assoc., 50: 69-79.
- Khan B, Iqbal A (2001). Production and composition of milk review. Pakist. J. Agric. Sci., 38 (3-4): 63-67
- Khan M Z, Khan A (2006). Basic facts of mastitis in dairy animals: A review; Pakist. Vet. J., pp 204-208.
- Khanna N D (1986). Camel as a milch animal. Indian Farming, 36(5): 39-40
- Kloos W E, Schleifer K H (1986). Staphylococcus. In: Sneath P. H. A., Mair N. S., Sharpe M. E. and Holt J. G. (eds), Bergey's Manual of Systematic Bacteriology, Vol.2. Baltimore, Hong Kong, London, Sydney. Williams and Wilkins, pp. 1013 – 1035
- Knoess K H (1977). The camel as a meat and milk animal. World Anim. Rev., 22: 3-8
- LCNRDB (2005). Livestock Crop and Natural Resources Development Bureau of Afar regional state, Ethiopia. Livestock, Crop and Natural Resources Development Bureau (LCNRDB)
- Makovec J A, Ruegg P L (2003). Results of milk samples submitted for microbiological examination in Wisconsin rom 1994-2001. J. Dairy Sci., 86: 3466-3472.
- Matofari J W, Mario Y, Mwatha E W, Okemo P O (2003). Microorganisms associated with subclinical mastitis in Kenyan camels (Camelusdromedarius). J. Trop. Microbiol., 2: 11-16.
- Matofari J W, Younan M, Nanua J N, Mwatha E W (2005). Microorganisms associated with sub-clinical mastitis and their impact on milk production in camels (Camelusdromedarius) in semi-arid lands of Northern Kenya, Internl. J. Agri and Rura Develop., 17: 182–187.
- McDonald J S (1997). streptococcal and staphylococcal mastitis. In Jarret, J. (ed): the veterinary clinics of North America. Large animals practice. Symposiumon bovine mastitis. W.B. Sounders Co, pp 269-285.
- Megersa B, Regassa A, Kumsa B, Abunna F (2008). Performance of camels (Camelus dromedrius) kept by pastoralists with different degree of experience in camel keeping in Borana, Southern Ethiopia. Anim. Sci. J., 79, 534-548
- Moore D A, Cullor JS, BonDurant RH, Sischo W M (1991). Preliminary field evidence for the association of clinical mastitis with altered interestrus interval in dairy cattle. Theriogenology36., 257–265.
- Muli M, David K, Peter K (2008). The camel milk industry in Kenya. Results of a study commissioned by SNV to explore the potential of Camel Milk from Isiolo District to access sustainable formal markets, Northern Keny.
- Mungube E O (2001). Management and economics of dairy cow mastitis in the urban and peri-urban areas of Addis- Ababa milk shed. Faculty of Veterinary Medicine, Addis Ababa University, Debre-zeit, Ethiopia, MSc.thesis.
- Murphy S C, Cranker K, SenykG F, Barbano D M, Saeman A I, Galton D M (1988). Influence of bovine mastitis on lipolysis and proteolysis in milk. J. Dairy Sci., 71: 65-69.
- NoletoA L, Bergdoll M S (1980). Staphylococcal enterotoxin production in the presence of non-enterotoxigenic staphylococci. Appl. Envir. Microbiol.,39: 1167 – 1171
- Nowak R M (1991). Walker's Mammals of the World.Vol II. Baltimore: John Hopkins University Press.
- Obeid AL, Bagadi H O, Mukthar M M (1996). Mastitis in Camelusdromedarues and the somatic cell content of camels’ milk. Res. Vet. Sci.,61:55-58
- OIE (2004). Multiannual animal disease status. Office International des Épizooties. (OIE) Available online at http://www.oie.int/hs2
- Oliveira C A F, Mestieri L, Santos M V, Moreno J F G, Spers A, Germano P M L (2000). Effect of microbiological characteristics of raw milk on the quality of whole milk powder.Braz. J. Microbio., 31: 95-98
- Pamela L, Douglas J (2002). Milk Quality and Mastitis Tests.University of Wisconsin, Madison.
- Patrick M, Kamau N, Stefania D, Christoph J, John W, Christophe L, Leo M (2013). Biodiversity and Enterotoxigenic Potential of Staphylococci Isolated from Raw and Spontaneously Fermented Camel Milk.Briti. Microbiol. Res. J., 3(2):128-138
- Quinn P J, Carter M E, Markey B, Carter G R (1994). Clinical veterinary microbiology. Wolf publishing, London, England. 327 – 344
- Quinn P J, Carter M E, Markey B K, Carter G R (1999). mastitis. In: clinical veterinary microbiology, Mosby international limited, London, Pp 327-344
- Qureshi M H (1986). The camel. A paper presented at FAO Seminar on Camel Production and Health, 20-23 October, Kuwait.
- Radostits O M, Blood D C, Gay G C (1994). Mastitis: Veterinary Medicine, a Textbook of the Disease of Cattle, Sheep, Pigs, Goats and Horse. 8th Edition, Bittler, Tindal, London, Pp 787-812.
- Radostits O M, Blood D C, Gay G C, Hinchcliff K W (2000). Mastitis. In: Veterinary Medicine, a Textbook of the Disease of Cattle, Sheep, Pigs, Goats and Horse. 9th Edition, W.B. Saunders Company Ltd., London, Pp 603-700.
- Radwan A I, Bekairi S I, Prasad P V S (1992). Serological and bacteriological study of brucellosis in camels in central Saudi Arabia. Rev. Sci. Tech. Off. Int. Epiz.11, 837 – 844.
- Ramet, J P (1989). Bay region agricultural development project: Camel milk component Mission report. Rome, IFAD. 29pp.
- Riyadh S A, Faris A, Moez A, Mohammad A A,Ali M A, Mansour F H (2011). Actors Influencing the Prevalence of Subclinical Mastitis in Lactating Dromedary Camels in Riyadh Region, Saudi Arabia. Trop. Anim. Hlth and Pro.(Inpress).
- Saad N M, ThabetA E R (1993). Bacteriological quality of camel's milk with special reference to mastitis. AssiutVet. Med. J.,28, 194 – 199.
- Satu p (2003). Review article Indicators of inflammation in the diagnosis of mastitis. University of Helsinki, Faculty of Veterinary Medicine, Department of Clinical Veterinary Sciences, Saari Unit, 04920 Saarentaus, Finland.
- Schalm O W, E J Carroll, Jain N C (1971). Bovine mastitis. Lea and Febinger, Philadelphia, USA.
- SchukkenY H, Grommers D, Van de Geer A, and Brand M (1989). Incidence of clinical mastitis on farms with low somatic cell counts in bulk milk. Vet. Res., 125:60-64.
- Schwartz H J (1986). The potential of the camel (Camelusdromedarius) as a transport and drought animal. FAO-MINEADEP Workshop on “Camels” Kuwait.
- Schwartz H J (1992). Productive performance and productivity of dromedaries (Camelus dromedarius). Anim Res Dev., 35: 86–89
- Schwartz H J, Dioili M (1992).The One Humped Camel in East Africa. A pictorial guides to disease, health care and management. Josef, FR Margraf, Germany. PP 1-10.
- Schwartz H J, Walsh M G H (1992). The productive potential of the camel. In: Schwartz H.J., Dioli M. (Eds.) The One-Humped Camel (Camelus dromedarius) in Eastern Africa: A pictorial guide to diseases, health care and management, pp. 30- 61. Verlag Josef Margraf, Weikersheim, Germany.
- Semereab T, Molla B (2001). Bacteriological quality of raw milk of camel (Camelus dromedarius) in Afar region (Ethiopia). J. Camel Pract. Res., 8, 51 – 54.
- Sena D S, Kumar R, Sahani M S (2000). Detection of subclinical mastitis in camels.J. Camel Pract. Res., 7: 203 – 204.
- Sena D S, Mal G, Kumar R, Sahani M S (2001). A preliminary study of prevalence of mastitis in camel, J. Appl. Anim. Res., 20: 27–31.
- Sharif A, Umer M, Muhammad G (2009). Mastitis Control in Dairy Production Pak. J. Agri Sci., 5: 102–105.
- Sibtain A, Muhammad Y, Muhammad Q B,Ghulam M, Li-Guo Y, Muhammad K K, Muhammad T (2011).Factors associated with prevalence and major bacterial causes of mastitis in dromedary camels (Camelusdromedarius) under different production systems; Trop Anim Healt Prod. Accessible at: www.pvj. com. pk.
- Simenew K, Dejen T, Tesfaye S, Fekadu R., Tesfu K, and Fufa D (2013). Characterization of Camel Production System in Afar Pastoralists, North East Ethiopia. Asia. J. Agri. Sci.,5(2): 16-24.
- SordilloL M (2005). Factors affecting mammary gland immunity and mastitis susceptibility, Livest. Prod. Sci., 98: 89-99.
- SPSS (statistical procedure for social science) (2007). SPSS (Version 16), BI survey tips statistical procedure for social science (SPSS) Inc. Chicago, USA.
- Tezera G (1998). Characterization of camel husbandry practice and camel milk and meat utilization in Shinille and Jijiga Zone of Somali National Regional State, MSc thesis, Alemaya University of Agriculture, Dire Dawa, Ethiopia.
- Thrusfield, M (1995). Veterinary Epidemiology. 2nd ed. London: Blackwell Science Ltd., pp 178-198.
- Tibary A, Anouassi A (2000). Lactation and udder disease. In: L. Skidmore and G.P. Adams (Eds.) Recent Advances in Camelid Reproduction. International veterinary Information Service. Available online at (www.ivis.org).
- TreeceJ M, Morse G E, Levy C (1966). Lipid analysis of bovine teat canal keratin.J. Dairy Sci., 49: 1240.
- Tura I, Kuria G, Walaga H, Lesuper J (2010). Camel Breeding Management among the Somali, Sakuye, Gabbra and Rendille Pastoralists of Northern Kenya. Kenya Agricultural Research Institute, Tropentag, September 14-16, 2010, Zurich, Kenya.
- Tuteja F C, Dixit S K, Ghorui S K, Deen A, Sahani M S (2003). Prevalence, characterisation and antibiotic sensitivity of intramammary infections in camel.J. Camel Pract. Res.,10: 69-77.
- Valeeva N I, Meuwissen M P M, Oude Lansink A G J M Huirne R B M (2005). Improving food safety within the dairy chain: an application of conjoint analysis. J. Dairy. Sci., 88: 1601-1612.
- Wardeh M (2004). Classification of the dromedary camels.J. Camel Sci., 1:1-7.
- Wernery U (1992). The prevalence of salmonella infections in camels (Camelusdromedarius) in the United Arab Emirates. Brit.Vet. J., 148(5): 445 – 450.
- Wernery U, Johnson B, Becker H, Märtlbauer E (2002). Microbiological status of raw dromedary milk.J. Camel. Pract. Res., 9: 1 – 4.
- WHO (2005). Drug-resistant Salmonella. Fact Sheet No 139. World Health Organization (WHO) Available online at http://www.who.int/ mediacentre/factsheets/fs139/en/print.html.
- Woubit S, Bayleyegn M, Bonnet P, Jean- Baptiste S (2001). Camel (Camelusdromedarius) mastitis in Borena lowland pastoral area, southwestern Ethiopia, Revue Elev. Medic. Vet. Trop., 54: 207-212.
- Yagil R (1985). The desert camel. Comparative physiological adaptations. S. Kanger AG, Switzerland, pp 109.
- Yayneshet T, Kelemework T (2004). Indigenous Rangeland resources and Conflict Management by the North Afar Pastoral Groups in Ethiopia.
- Yohannes M, Zeleke M, Getachew G (2007). Potentials of camel production in Babilie and Kebribeyahworedas of the Jijiga Zone, Somali Region, Ethiopia, Pp 9-10.
- Younan M (2004). Milk hygiene and udder health. In: Farah, Z. and Fischer, A. (eds): Milk and meat from the camel - Handbook on products and processing. vdf Hochschulverlag, Zürich, Switzerland, pp. 67 – 76.
- Younan M, Ali Z, Bornestein S, Muller W (2001). Application of the California mastitis test in intramammary Streptococcus agalactiae and Staphylococcus aureus infections of camels (Camelusdromedarius) in Kenya, Prev. Vet. Medi.,51: 307-316.
- Younas M, Iqbal A (2001). Milk composition of Pakistani camel (Camelusdromedarious) kept under station/farmer's conditions. Emir. J. Agric. Sci., 13: 7-10.