Information
Journal Policies
Identification and Molecular Characterization of Lumpy Skin Disease Virus in East Hararghe and East Shoa Zone, Oromia Regional State
Asmelash Tassew1, Aster Assefa2, Essayas Gelaye2, Berecha Bayisa2, Mulugeta Ftiwi1
2.National Veterinary Institute, Bishoftu, Ethiopia.
Copyright :© 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Lumpy skin disease virus of the genus Capripoxvirus is a causative agent for Lumpy skin disease in cattle. In Ethiopia, a live attenuated vaccine strain (KS1-O180) has been used for immunization of cattle. A cross sectional study was conducted from December 2016 to May 2017 in selected districts of East Oromia Region in order to isolate and molecularly characterize the virus collected from clinically sick cattle. Purposive sampling technique was used to collect tissue specimens from Gursum and Sodere areas from cattle showing generalized circumscribed nodular skin lesions covering the entire body with deep eroded and crusted lesions. Virus isolation was conducted using Epithelial Bovine Skin origin cell line cultures. The isolates were then genotyped using classical and real-time detection by polymerase chain reaction methods, retrospectively. Furthermore, the RPO30 full gene (606 nucleotides) was sequenced and phylogenetic tree was reconstructed and all the current Lumpy skin disease virus isolates clustered under the Lumpy skin disease virus group. Out of the clinically sick cattle, Lumpy skin disease with 5.69%, 0.34% and 5.97% morbidity, mortality and case fatality rates was observed respectively. Skin biopsies poxvirus cytopathic effect was observed in infected cell line within five days of post- inoculation after one blind passage. On the second passage cytopathic effect was observed within three days of post inoculation without any blind passage. The RPO30 gene sequence alignment of the current field isolates with the previously characterized Ethiopian Lumpy skin disease virus revealed single nucleotide and amino acid variation at position 41A/C and 14N/T, respectively. Similarly, a single nucleotide and amino acid mutation observed at position 292 T/C and 98Y/P between the outbreak isolates and vaccine strain respectively. This study showed that the vaccine strain was genetically distinct from the field isolates. Therefore, further molecular genotyping and sequencing of the circulating Capripox virus isolates is recommended to discover the antigenic variation of circulating virulent field strains with the vaccinal strain.
Capripox virus, Ethiopia, Isolation, Lumpy skin disease, Molecular, Outbreak,Animal and Veterinary Sciences
1. Introduction
Lumpy skin disease (LSD) is a pox disease of cattle and is characterized by fever, nodules on the skin, lesions in the mouth, pharynx and respiratory tract, emaciation, enlarged lymph nodes, edema of the skin, and sometimes death (Carn and Kitching, 1995; Davies et al.,1971; OIE, 2010; Gari et al., 2011). The disease is one of the most important viral diseases of cattle, causing loss of condition in infected animals and permanent damage to hides. The most effective route of transmission is mechanical via biting flies. The incidence of LSD is high during wet seasons when populations of the flies are abundant and decreases or ceases during the dry season (Gari et al., 2012).
LSD has a different geographical distribution from that of sheep- and goat-pox, suggesting that cattle strains of capripoxvirus do not infect or transmit between sheep and goats (OIE, 2010; Ahmed and Kawther, 2008). The disease was first observed in 1929 in northern Rhodesia (currently Zambia) and rapidly spread north and south. It now occurs in most of Africa (except Libya, Algeria, Morocco and Tunisia) and much of the Middle East (Tuppurainen and Oura, 2012).
The World Organization for Animal Health (OIE) categorizes LSD as notifiable because of the substantial economic impact of an outbreak. The disease is more severe in cows at peak lactation and causes a sharp drop in milk yield, often leading to secondary bacterial mastitis. Temporary or permanent infertility may occur in cows and bulls. The emaciation of infected animals and a convalescence period lasting for several months causes a decreased growth rate in beef cattle (Tuppurainen and Oura, 2012; Brenner et al., 2006). The morbidity and mortality of the disease vary considerably, depending on the breed and immunological status of the cattle population and the insect vectors involved in transmission. In a few outbreaks morbidity has been reported as more than 50%, although the mortality rates were usually less than 10%. The abortion rate in pregnant cows may range from 1% to 7% (Radostits et al., 2007; Vorster and Mapham, 2008).
In Ethiopia, LSD was first observed in 1983 in the north-western part of the country (south-west of Lake Tana) (Mebratu et al., 1984). The disease has now spread to almost all regions and agro-ecological zones of the country. Because of the wide distribution of the disease and the size and structure of the cattle population in Ethiopia, it is likely that LSD is one of the most economically important livestock diseases in the country (Gari et al., 2012).
The effective control or eradication of LSD in endemic and non-endemic areas requires rapid and accurate diagnostic methods to confirm a presumptive diagnosis. This requires adequate financial, infrastructural, and human resources and adequate information system. However, under the current Ethiopian situation, these control strategies could not be implemented to control and eradicate the disease. Therefore, control measures through vaccination and restriction animal movement remain the most practical option in the country.
However, there were different published and un published reports about incomplete protection of existing LSD vaccines in Ethiopia (Ayelet et al., 2014; Gelaye et al., 2015, Girma, 2015) showed that the presence of genetic variation between NVI LSD vaccine strain and field isolates based G-protein-coupled chemokine receptor (GPCR) gene analysis. Such finding possibly indicated the progress of genetic change of field LSD strains circulating in the country. Continuous studies on LSD outbreaks investigation and genetic characterization need to understand the genetic variation of field isolates. Furthermore, detailed molecular characterization of the LSD virus isolates and the vaccine strain could help to design strategy to fulfill existing incomplete protection.
Therefore, the objectives of the study were:
1. To determine the occurrence of LSD in the study area.
2. To isolate field LSD virus strains on ESH-L cell line and molecularly characterize the isolates.
2. Materials And Methods
The study was conducted in East Hararghe zone of Gursum district from three peasant association (Awdal, Qebso and Harobati) and Central Ethiopia East Shewa Zone of Adama district (Sodere) From one private fattning farm of Oromia Regional State from November 2016 to May 2017. Gursum district (Funyan Bira town) is located at 600 km East of Addis Ababa, the capital city of Ethiopia. Geographically, the district lies between 9° 07ʹ and 9° 32ʹ North latitudes and 42° 17ʹ and 42° 38'E longitudes. The altitude of the district ranges from 1200 to 2938 m above sea level with the annual rain fall of 650 to 750 mm and the mean annual minimum and maximum temperature of 18 and 25°C, respectively. Gursum is bordered in the east by Somali regional state, in the west by Harari regional state, in the north by Jarso district and in the south by Babile district. It is inhabited by a human population of about 168476 people (CSA, 2013). The district is divided into 3 agro-ecological zones: highland (5%), midland (45%), and lowland (50%). The area has short rainy season (March to April) and long rainy season (June to August) according to (Gursum Livestok and Fishery Development Office, 2015).
While Central Ethiopia has a bimodal rainfall season: the long rain fall season from late June to late November and short season from February to April, with mean annual Rainfall range 450mm to 1,000mm and temperature range of 17°C to 30°C. Also have three agro-climatic zones; midland, lowland and highland zones representing 70%, 25% and 5% of the total area respectively (East Shewa Agricultural Zone, 2011). Study area was selected based on the occurrence of active cases to obtaining representative sample.
Active outbreaks were assessed by frequent observation and communicating Zonal Regional Laboratories and district animal health workers. In searching outbreak, it was done together with veterinary professionals who are working in the district of veterinary clinic. Field investigations were conducted based on information on previous vaccination history and clinical signs where an active outbreak of LSD was reported.
A structured questionnaire format was prepared to interview individual owners of cattle. The investigation also assessed the occurrence of the disease like day of outbreak, number of cattle at risk, number of cases, death and history of vaccination was gathered by interviewing cattle owners and animal health professional. The data was carefully recorded on a designed format.
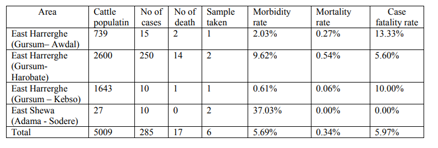
A total of 5009 cattle were assessed and cattle that showed clinical signs of pox like skin lesion were targeted for this study. All cattle included in the study were indigenous Zebu breed of both sexes and managed under extensive farming system by small holder farmers holding 2 to 30 cattle.
During visit, visual inspection was made to observe the presence of typical clinical sign of lumpy skin disease and a detailed physical examination was done on sick cattle. Six skin nodules were collected from representative sick cattle (two from Adama & four from Gursum) to identify the causative agents. According to the procedures indicated in OIE (2014), samples for virus isolation and antigen detection were collected from clinically sick animals. A total of 6 skin nodules from representative cattle which had developed severe clinical sign of the disease were taken aseptically by washing and cleaning the area and removing the hairs with sterile scalpel blade. Tissue samples were placed in the sterilized universal bottle containing antibiotics and antifungal and transported to the NVI virology laboratory maintaining cold chain system. Then, the tissue samples were stored at - 20°C.
The skin biopsy samples were thawed at room temperature and washed three times using sterile phosphate buffer saline (PBS) at a pH of 7.2 containing antibiotics and antifungal in Bio-safety cabinet class II. Tissue homogenates (10%W/v) were prepared in a sterile PBS. The tissue suspension was centrifuged at, 4000rpm/10min. The supernatant was collected and preserved at -200C (OIE, 2014).
Following centrifugation, 0.5ml of homogenate supernatant was inoculated on confluent monolayer ESH-L (source AU-PANVAC) cell line in a 25cm2 tissue culture flasks. Ten ml of Minimum Essential Medium (Sigma-Aldrich) supplemented with 5% fetal calf serum (Guich) was added and the cultures were incubated at 370C at 5% CO2. The medium was changed after 48 hours by maintenance media. Medium was supplemented with 2% calf serum. Cell cultures were observed daily for the development of CaPV specific cytopatic effects (CPE).A sample was considered negative when no CPE was observed after three blind passages. Cell culture that showed CPE was freezed at - 20°C and thawed three times at room temperature to release the virus particle. Finally, the virus suspension was stored at -20°C until processed for DNA detection (OIE, 2014).
DNA extraction was conducted from tissue homogenate and infected cell culture suspension, using DNeasyR Bllood and Tissue kit (Qiagen, Germany) Following the manufacturer’s instruction, 200 μl of tissue homogenate or cell culture suspension was transferred in to a 1.5ml eppendorf tube, and 20μl,Proteinase Kwas added and mixed well.,200 μl,of AL Bluffer was added in to virus suspension and gently mixed by overtaxing and incubated at 56°C for 10min in a water bath.200μl, of 95% ethanol was added and mixed thoroughly, The mixture was transferred in to DNeasy min column in 2ml collection tube and centrifuged at 8000rpm for 1min.The spin column was transferred in to a new 2ml collection tube and 500 μl of AW1 was added and centrifuged at 8000 rpm for 1min. The collection tube was discarded and the minispin column was placed in a new 2ml collection tube and 500μlof AW2 was added and centrifuged at 14000rpm for 3min.The minspin column transferred carefully in to new1.5ml of micro centrifuge tube and 50 μl elution buffer was added and incubated for 1min at room temperature and centrifuge at 14000rpm for 2min. The spin column was discarded and the eluted DNA was labeled and stored at -200C freezer until tested by PCR.
A PCR was carried out to detect the capripox virus genome using capripox virus-specific primers of SpGpRNAPol Forward, 5’TAGGTG ATTTTGGTCTAGC- TACGGA 3'and SPGp RNAPol-Reverse 5' AGTGATTAGGTGGTG TATTATTTTCC 3' previously designed by Lamien et al. (2011), PCR was done in a reaction volume of 2 μl containing supper mix 10 µl, temple DNA 3µl, Forward primer 2 μl, Revers primer 2μl and RNAse free water 3µl. The PCR tube was transferred in to a thermal cycler and amplification was conducted. Following the program: initial denaturation at 95°C for 4minutes followed by 40cycles at 95°C /30sec, annealing at 50°c /30sec and extension at 72°C/30sec, and final extension at 72°C /5min. Aliquots of PCR products were analyzed using 3% agarose gel stained with GelRed (Biotium, inc.) at 100V for 1h. The PCR results were considered positive for LSD DNA when 172bp observed.
Agarose gel electrophoresis provides a means of analyzing DNA by separating molecules based on size. Amplified products were analyzed by agarose gel electrophoresis as described by Mangana- Vougiouka et al. (1999) to confirm the presence of DNA. 1.5% Agarose gels prepared in Tris/Acetate/EDTA (TAE). Amplified products were analyzed with a component of 1µl loading buffer with gel red, and 5µl PCR product loaded to wells in previously prepared gel and run at 100volt/1hr. Parallel with DNA molecular weight marker in electrophoresis apparatus until the DNA samples have migrated a sufficient distance through the gel. DNA bands were visualized using UV transilluminator at a wave length of 590nm, and positive results were confirmed according to the size of the bands formed on agarose gel. The PCR results were considered as positive for LSDV when 172bp observed.
The recently developed species specific real-time PCR method using unlabeled snapback primer and dsDNA intercalating dye assay targeting the CaPVRPO30 gene was used to confirm the capripoxvirus identity of the field isolates and determine the genotype (Gelaye et al., 2013). Real-time PCR was performed at the Molecular Biology Laboratory of NVI using the amplification primers and PCR protocol described by Gelaye et al., (2013). Briefly, the PCR was set up in a reaction volume of 20 µL; where 4.84 µl of RNAase free water, 2µL of forward primer (CP-HRM-sb gene sequence 5’GGTGTAGTACGTATAAGATTATC GTATAGAAA- CAAGCCTTTA3’), 0.16µL reverse primer (CP-HRM1 gene sequence 5’AATTTCTTTCTCT- GTTCCATTTG3'), 10µL of SsoFast EvaGreen Super mix (BioRad) and 3 µL sample template. PCR was performed with an initial denaturing step at 95°C for 3minutes, followed by 45 cycles at 95°C for 15sec and 58°C for 80 sec using Low Profile Hard-Shell® 8-well PCR strips (BioRad). Then product was then denatured at 95°C for 1mimute (held for 1minute), cooled to 40°C (held for 1minute), and heated continuously at 0.5°C/10sec with fluorescence acquisition from 45°C to 85°C. Finally, pair of melting temperature each for snapback tail and full amplicon was recorded as LSDV (51°C/73.5°C), GTPV (56°C/72.5°C), and SPPV (52°C/72.5°C) for genotyping of the tested isolate.
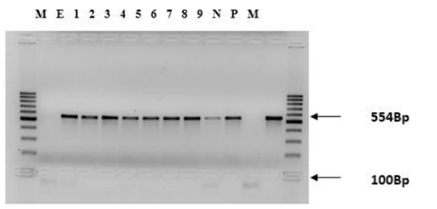
Two sets of primers for the RPO30 gene were used for the amplification of the RPO30 gene as briefly described by Gelaye et al., (2015). The primers were designed to amplify two overlapping fragments with a size of 554 and 520bp. The aim of using these overlapping primers was to generate the full length RPO30 gene. PCR was conducted in reaction volume of 20 µL containing 2µL forward primer (gene sequence CPRPO30-OL1F5’CAGCTGTTTGT TTACA-TTTGATTTTT3’, CPRPO30-OL1R 5’ TCGTATAGAAACAAGCCTTTAATAGA3’, 2µL reveres primer (gene sequence CPRPO30- OL2F 5’TTTGAACACATTTTATTCCAAAA AG3’ CPRPO30-OL2R 5’AACCTACATGCAT AAACAGAAGC 3’), 2.5µL dNTPs, 2.5 µL 10x PCR Buffer (Qiagen), 0.5 µL Taq polymerase (Qiagen), 5.5µL RNase free water and 5 µL template DNA. The initial denaturation at 95ºC for 4minutes was followed by 40 cycles at 95°C for 30ses, 55°C for 30ses and 72°C for 45ses and then the final extension at 72°C for 7minutes. Aliquots of PCR products were checked using electrophoresis on a 1.5% agarose gel stained with GelRed (Biotium, inc.) for 1h at 100v.
The positive PCR products of the amplified RPO30 gene were purified using the Wizard SV Gel and PCR clean-up system kit (Promega, Germany). The concentration of the purified PCR product was quantified using the NanoDrop 200°c spectrometer (Thermo Scientific, USA). The concentration of each purified product was adjusted and prepared according to the instruction recommended by the sequencing providing company. The purified PCR products were mixed with the sequencing primers and submitted for sequencing to the commercially sequencing LGC Genomics (Berlin, Germany).
The raw sequence data were edited and fragments were assembled using Vector NTI Advance™ 11.5 software (Invitrogen, Carlsbad, CA, USA). For each isolate, the fragments produced with both sets of overlapping primers of the RPO30 gene were edited and assembled together and the clean gene sequence was extracted. Multiple sequence alignments were performed using the ClustalW algorithm implemented in BioEdit software package to compare the RPO30 gene of the outbreak isolates and the reference strain. For comparative studies, blastn was used to collect additional Capripoxvirus RPO30 gene sequences from GenBank for inclusion in the data set. For construction of phylogenetic tree, multiple sequence alignments were performed to align the sequences as codons using the Muscle algorithm in MEGA6 (Tamura et al., 2013). The Neighbor-Joining algorithm was used with the maximum composite likelihood nucleotide substitution model with the pair wise deletion option was used. For construction of phylogenetic tree, 1000 bootstrap replicate was used.
Data obtained from all field and laboratory investigations was coded and stored in Excel spread sheets. Data collected during observation of clinical signs while investigating the outbreak, sample collection, virus isolation using cell culture, CaPV targeted gene amplification using classical and real-time PCR, sequence analysis and phylogenetic tree construction was analyzed separately using the different bioinformatics software’s. The analyzed data was interpreted and presented into biological terms.
3. Results
The common clinical signs observed in cattle affected by suspected LSD virus were fever, depression, loss of appetite; circumscribed nodules on the skin with different sizes and enlargement of superficial lymph nodes, lacrimation, nasal discharges and decrease in body weight were prominent signs of the disease.
A total of 5009 local Zebu breed cattle were investigated. According to the information obtained from cattle owners, veterinarians and animal health assistants working in the outbreak areas, a total of 285 cattle were affected by LSD, out of which 17 cattle were died, with 5.69%, 0.34% and 5.97% morbidity, mortality and case fatality rates respectively (Table1).
All collected 6 representative skin biopsies characteristic poxvirus CPE was observed in infected ESH-L cells line within five days of post- inoculation after one blind passage. On the second passage CPE was observed within three days of post inoculation without any blind passage.
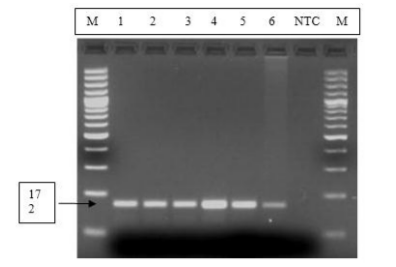
The extracted DNA of the 6 poxvirus isolated was further confirmed by amplification of the virus gene using gene -specific primer. PCR products on a 3% high-resolution agarose gel DNA fragment of 172bp product size for LSDV was observed in all the six tested samples, and none amplification seen on the non-template control.
Lanes M: DNA ladder; lane NTC: Negative Template Control; Lanes 1, 2, 3, and 4 represent positive samples from Gursum; Lanes 5 and 6 represent positive samples from Adama.
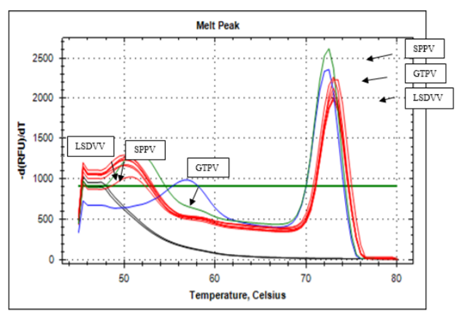
The presence of CaPV on samples collected from cattle was confirmed and further genotyped using gene specific real time PCR method: the snapback assay targeting the RPO30 gene. The results showed that all new six field isolates recovered from cattle were genotyped as LSDV with melting temperatures of 51°C and 73.5°C for snapback and full length amplicons, respectively (Figure 3). The vaccine strain KS1-O180 used for cattle was also tested and confirmed to be a LSDV. There was a 100% agreement between the results of the classical PCR and the real time PCR methods. However, GTPV and SPPV had initial melting peaks of 560C and 51°C as well as a second peak of 72°C respectively.
The figure shows the two melting peaks where the new six field isolates were genotyped as LSDV. The three genotypes of sheeppox virus, Goatpox virus, and Lumpy skin disease virus were included in the PCR reaction as positive controls.
Six new isolates, two previously isolated samples and one vaccinal strain of PCR product of RPO30 gene were run by gel electrophoresis on a 1.5% agaros gel for 1hour at 100v by 100bp molecular weight marker. Where, Lane M: DNA molecular markers (Fermentas); Lane E: DNA extraction control; Lane 1-6: LSDV current field samples, Lane 7 & 8: LSDV previously isolated field samples, Lane 9: vaccinal strain and Lane P: positive LSDV control.
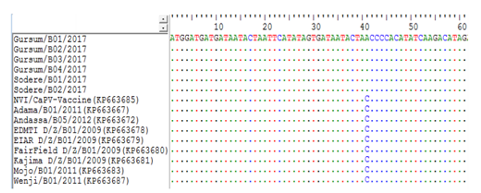
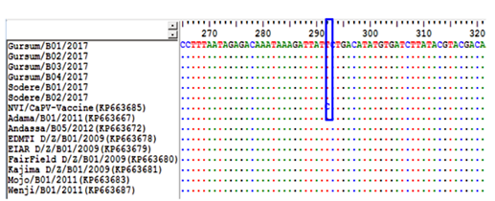
To determine if there was any difference between the newly collected six capripoxvirus isolates based on the geographical area of sample collection, multiple sequence alignments of the RPO30 gene sequences was performed. The sequence analysis showed that no significant nucleotide variation among the field isolates included in the current study. However, there is a single nucleotide mutation exists between the new and the previously characterized field Ethiopian LSDV isolates causing A/C change at nucleotide position 41 (Figure 5). Similarly, the deduced amino acid sequences of the RPO30 gene of the new and old Ethiopian LSDV isolates also revealed a single amino acid variation N/T at residue position 14.
A unique nucleotide variation (A/C) observed between the new and old LSDV isolates at nucleotide position 41, is highlighted in the box.
Identical nucleotides are indicated with dots.
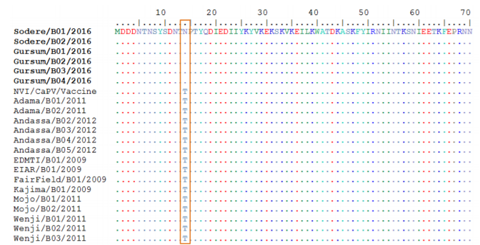
Amino acid sequence variations observed between the current and old LSDV isolates with vaccinal sain at codon position Y98P was highlighted in the box, Identical amino acids are indicated with dots.
To determine whether the LSD outbreaks in vaccinated cattle could be attributed to a residual pathogencity of the vaccine used, multiple sequence alignments of the RPO30 A unique nucleotide variation observed between the field LSDV isolates and the vaccine strain at nucleotide position 292T/C, is highlighted in the box, Identical nucleotides are indicated with dot.
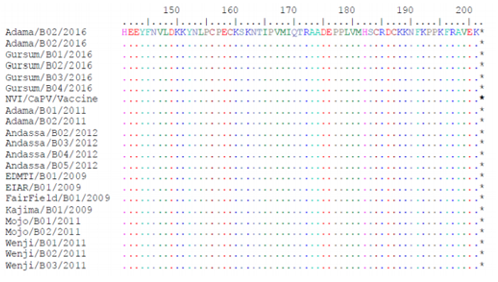
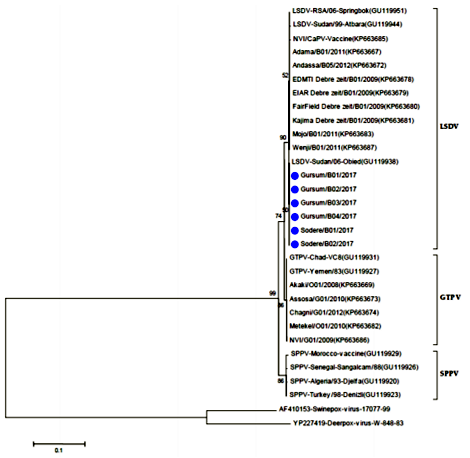
Phylogenetic reconstruction was performed to determine the genetic relationship among new LSDV isolates as well as other CaPV isolates retrieved from the GenBank. The analysis of the full length RPO30 gene sequence of all current six field isolates collected from diseased cattle classified under the LSD virus group as shown in the figure (6 & 7). The phylogenetic tree was fully in agreement with the genotyping result of the classical and real-time PCR analysis.
A number of six Ethiopian outbreak isolates, the local vaccine strain and the sequences retrieved from the GenBank were used. The Neighbor-Joining method with the maximum composite likelihood nucleotide substitution model and the pair wise deletion option was compute using MEGA6. The percentage bootstrap scores above 50% (out of 1000 replicates) are shown next to the branches. The homologue gene sequence from one Deer poxvirus and one Swine poxvirus isolates retrieved from the GenBank were used as out-group. The six isolates sequenced in this study are marked on blue tree (figure 8,9,10).
4. Discussion
The findings of clinical signs, virus isolation and PCR diagnosis confirmed that the outbreaks were caused by LSDV. In support of the present study, several authors reported the occurrence of LSD outbreaks in different parts of Ethiopia on different periods (Gelaye et al., 2015; Girma, 2015; Gelagay et al., 2014; Gelagay et al., 2013, Gari et al., 2010). Moreover, Gelagay et al (2013) stated that LSD had been occurring as epidemic since 2008.
Morbidity of the LSD outbreak during the present study were 5.69% (n=285) out of the investigated 5009 cattle. Morbidity of LSD varies from 3% to 85% (Babiuk et al., 2008; Tuppurainen and Oura, 2012). Genetic difference, immunity status, geography location and climate and virulence of virus strain were raised for morbidity variation. In the contrary, Gelagay et al., (2013) reported nearly similar morbidity rates of 22.5% and 25.9% for cross and local breeds, respectively. Although the present average morbidity rate was lower than the report of Gelagaye et al., (2013), the relative higher morbidity (37.03%) was estimated in Adama-Sodere Woreda of East Shoa Zone. Higher morbidity in the East Shoa as compared to 5.52% in East Harerghe Zone might be due to humid and hot climate which is favorable for the abundance of the vectors. The mortality rate and case fatality rate were 0.34% and 5.97% respectively. The present study is in agreement with the conclusion that mortality rate never exceeds 3% (Babiuk et al., 2008; Tuppurainen and Oura, 2012). Lower mortality rate of 0.34% was in favor of knowledge that the local Zebu breeds are less susceptible than cross or exotic breeds (Davies, 1991; Woods, 1988).
The clinical signs observed on investigated sick animals were depression, lacrimation, nasal discharge, loss of body condition and circumscribed skin nodules over the skin. These clinical manifestation were also recorded as clinical signs of LSD affected animals (Coetzer, 2004; Grooms, 2005; Alaa 2008; Gari et al., 2010; Salib and Osman, 2011; Gelagay et al., 2013; Gelaye et al., 2015). Swelling limbs, lameness and reluctant involvement of epithelial cells of digestive and abortion were reported on cross breeds (Gelagay et al., 2014; Girma, 2015). However, these signs were not observed during the current LSD outbreaks. Such variation of clinical signs could be attributed to genetic difference.
As Babiuk et al., (2008) reviewed, primary lamb kidney or primary lamb testis cells are the most commonly used cells for LSDV isolation. Because of disadvantages of primary cell such as constant establishment of new cultures, cell lot variation, and contamination with extraneous agents, a lamb testis secondary cell line (OA3.Ts) has been evaluated as a replacement for primary cells (Babiuk et al., 2007). On the other hand, several authors isolated LSDV from skin samples using Vero cell line in NVI Virology Laboratory (Gelagay et al., 2013, Gelagay et al., 2014; Gelaye et al., 2015; Girma 2015). However, 3 out of 10` (Gelagay et al., 2013) and five out of 31 (Girma, 2015) skin biopsies taken from cattle with typical LSD clinical signs were reported as unsuccessful isolation using Vero cell line. Gelaye et al. (2015) also indicated that some samples required blind passages for isolation. In contrary, in the present study, six isolates were obtained from all six skin biopsies using ESH-L cell line and all isolates were identified as LSDV after sequential diagnosis using convention PCR and real-time PCR. The CPEs of LSDV on cell line were characterized by rounding of single cells, aggregation of dead cells and destruction of monolayer. Therefore, as the above authors’ reports and the present findings, LSDV produces almost similar CPEs on both Vero cell and ESH-L cell lines. However, further experiment is needed to compare both cell lines for their suitability for routine LDSV isolation in the future.
Parameters influencing vaccination failures, including the real identity of the vaccine and the diversity of the CaPV isolates were investigated. The sequence analysis showed that no nucleotide variation among the field isolates included in the current study. However, there is a single nucleotide mutation exists between the new and the previously characterized field Ethiopian LSDV isolates causing A/C change at nucleotide position 41.
Similarly, the deduced amino acid sequences of the RPO30 gene of the new and old Ethiopian LSDV isolates also revealed a single amino acid variation N/T (threonine by asparagines) at residue position 14. Furthermore, amino acid sequence variations observed between the current and old LSDV isolates at position N14T; and between field isolates with vaccine strain at amino acid residue Y98P (proline by tyrosine) are highlighted in the box (figure 11), respectively. Identical amino acids are indicated with dots. On the other hand, multiple sequence alignments of the RPO30 gene showed that a sequence difference between the vaccine strain and the field isolates. A single nucleotide variation was found between the outbreak isolates and the vaccine strain T/C at nucleotide position 292. This sequence variation was also observed in the deduced amino acid sequences alignment where variation existed at residue 98 Y/P position. This result indicates that the isolates causing LSD disease in Ethiopia are different from the vaccine strain. This is in agreement with reports of Gelaye et al. (2015)
The results of questionnaire survey revealed that LSD outbreaks occurred in the study areas between 2 months and two years post vaccination. This is in agreement with reports of LSD vaccines associated with incomplete protection and adverse reactions in cattle post vaccination (Ali et al., 1990; Ayelet et al. 2013; Brenner et al. 2009; Eeva et al., 2014; Khalafalla et al., 1993; Omyma, 2008 Somasundaram, 2011 and Tamam, 2006). Ayelet et al. (2013) also reported that the Kenyan sheep pox vaccine strain used for the control of LSD in Ethiopia did not confer expected protection, and urge the need for investigation of vaccine failure including vaccine matching and alternative vaccine development.
5. Conclusion And Recommendations
LSD was found to be the major cattle health problem causing severe economic loss due to permanent damage to hides, a prolonged debilitating clinical course, reduced weight gain, temporary or permanent loss of milk production, temporary or permanent infertility or even sterility in bulls, and abortion of pregnant cows.
Molecular analysis indicated that there was no significant nucleotide variation among the current and previous field isolates included in the current study except a single nucleotide mutation and a single amino acid variation. On the other hand, a sequence difference was observed between the vaccine strain and the field isolates. Based on the above conclusion the following recommendations are forwarded.
1. Comparative studies of cell lines for the isolation of LSDV should be conducted,
2. Molecular characterization should be conducted to discover the variation of circulating virulent field strains with reference to vaccinal strain and
3. Further studies should be conducted to discover the effect of nucleotide and amino acid change on pathogenecity of virus and immunogenicity of the vaccine.
References
- Ahmed, W. and Kawther, S. (2008): Observations on lumpy skin disease in local Egyptian cows with emphasis on its impact on ovarian function. African Journal of Microbiology Resear, 2: 252–257.
- Alaa, A., Hatem, M., Khaled A. (2008): Polymerase chain reaction for rapid diagnosis of a recent lumpy skin disease virus incursion to Egypt. J. Arab Biotechnology, 11:293-302.
- Alexander, R., Plowright, W., and Haig D. (1957): Cytopathogenic agents associated with lumpy skin disease of cattle. Bulletin Epizootic Disease of Africa, 5: 489-492
- Ali, A. A., Esmat, M., Attia, H., Selim, A., and Abdel-Hamid, Y. M. (1990): Clinical and pathological studies of lumpy skin disease in Egypt. Veterinary Records, 127: 549–550.
- Anon (2008): Lumpy Skin Disease. In: USDA, A., NVSL (Ed.). Foreign Animal Disease. The Grey Book. Foreign Animal Disease Diagnostic Laboratory, Greenport, NY 11944.
- AUSVETPLAN (Australian Veterinary Emergency Plan) (2009): Disease strategy: lumpy skin disease (Version 3.0). Edition 3, Canberra, Primary Industries Ministerial Council (available at http://www.oie.int/ file admin/ Animal Health in the World/docs/pdf/ LSD12FINAL_27Jan09_. pdf)
- Ayelet, G., Abate, Y., Sisay, T., Nigussie, H., Gelaye, E., Jemberie, S. and Asmare, K. (2013): Lumpy skin disease: preliminary vaccine efficacy assessment and overview on outbreak impact in dairy cattle at DebreZeit, central Ethiopia. Antiviral Res., 98 (2), 261–265. doi:dx.doi.org/10.1016/j.antiviral.2013.02.008.
- Ayelet, G., Haftu, R., Jemberie, S., Belay, A., Gelaye, E., et al (2014): Lumpy skin disease in cattle in central Ethiopia: outbreak investigation and isolation and molecular detection of lumpy skin disease virus. Review. Science and technology. Office. International. Epizootic, 33 (3)877-87.
- Babiuk, S., Bowden, T., Boyle, D., Parkyn, G., and Copps, J. (2008a). Quantification of lumpy skin disease virus following experimental infection in cattle. Transboundary and Emerging Diseases, 55: 299-307.
- Babiuk, S., Bowden, T., Boyle, D., Wallace, D., and Kitching, R. (2008b): Capripoxviruses: Anemerging worldwide threat to sheep goats and cattle Transboundary and EmergingDiseases, 55: 263-272.
- Babiuk, S., Parkyn, G., Copps, J., Larence, J., Sabara, M., et al (2007): Evaluation of an ovine testis cell line (OA3.Ts) for propagation of capripoxvirus isolates and development of an immunostaining technique for viral plaque visualization. Journal of Veterinary Diagnostic Investigation, 19: 486–491.
- Barnard, B. J., Munz, E., Dumbbell, K., and Prozesky, L. (1994): Lumpy Skin Disease. In infectious disease of livestock with special reference to Southern Africa, edited by Coetzer, J. A. W., Thomas, G.R. and Tustin, R.C. Cape Town: Oxford University Press, Southern Africa, Pp:604-612.
- Barnard, B.J.H. (1997): Antibodies against some viruses of domestic animals in southern African wild animals. Onderstepoort Journal of Veterinary Research, 64(2):95-110
- Bennett, R.I. and Jpelaar, J. (2005): Updated estimates of the costs associated with 34 endemic livestock diseases in Great Britain: a note. J. Agric. Econ., 56: 135-144.
- Bertagnoli, S. and Séverac, B. (2010): Pox viruses. In: Lefèvre, P.C., Blancou, J., Chalmette, R., Uilenberg, G. (Eds.), Infectious and Parasitic diseases of Livestock. Lavoisier, Paris, Pp.367
- Beshahwured, S. (1991): Outbreak of Lumpy skin disease in and around Wolliso. DVM thesis. Faculty of Veterinary Medicine, Addis Ababa University, Debre- Zeit, Ethiopia.
- Binepal, Y., Ongadi, F., and Chepkwony, J. (2001): Alternative cell lines for the propagation of lumpy skin disease virus. Onderstepoort Journal of Veterinary Research, 68: 151-153
- Bowden, T. R., Babiuk, S. L., Parkyn, G. R., Copps, J. S., and Boyle, D. B. (2008). Capripox virus tissue tropism and shedding: A quantitative study in experimentally infected sheep and goats. Virology, 371: 380–393.
- Brenner, J., Bellaiche, M., Gross, E., Elad, D., Oved, Z., Haimovitz, M., Wasserman, A., Friedgut, O., Stram, Y., Bumbarov, V., and Yadin, H. (2009): Appearance of skin lesions in cattle populations vaccinated against lumpy skin disease: statutory challenge. Vaccine, 27, 1500–1503.
- Brenner, J., Haimovitz, M., Oron, E., Stram, Y., Fridgut, O., Bumbarov, V., Kuznetzova, L., Oved, Z., Waserman, A., Garazzi, S., Perl, S., Lahav, D., Edery, N., Yadin, H. (2006): Lumpy skin disease in a large dairy herd in Israel; Journal of Veterinary Medicine,61:73-77.
- Cameron, C., Hota-Mitchell, S., Chen, L., Barret, J., Cao, J. X., et al (1999): The Complete DNA Sequence of Myxoma Virus. Virology, 264: 298-318.
- Carn, V. M. (1993): Control of capripox virus infections. Vaccine, 11(13): 1275-1579.
- Carn, V. M. and Kitching, R. P. (1995a): An investigation of possible routes of transmission of lumpy skin disease virus (Neethling). Epidemiology and Infection, 114: 219–226.
- CFSPH, (2008): The Center for Food Security and Public Health, Iowa State University, College of Veterinary Medicine and Institution of International cooperation in Animal Biologics, an OIE collaborating center.
- CFSPH, (2011): Center for Food Security and Public health. Iowa State University, College of Veterinary Medicine.
- Chihota, C., Rennie, L. S., Kitching, R. P., and Mellor, P. S. (2001): Mechanical transmission of lumpy skin disease virus by Aedesaegypti (Diptera: Culicidae). Epidemiology and Infection, 126: 317-321.
- Coakley, W. and Capstick, P. B. (1961): Protection of cattle against lumpy skin disease. Factors affecting small scale production of tissue culture propagated virus vaccine. Research in Veterinary Science, 2: 369–371.
- Coetzer, J.A.W. (2004): Lumpy skin disease. In: Infectious Diseases of Livestock. 2nd edition. Coetzer, J.A.W. and Justin, R.C. (eds). Oxford University Press, Cape Town, South Africa. Pp: 1268-1276.
- CSA, (2011). Agricultural sample survey 2010/2011, Report on livestock and livestock characteristics. Central Statistical Agency of Ethiopia. Statistical Bulletin, 505.
- Damaso, C. R., Esposito, J. J., Condit, R. C., and Moussatché, N. (2000): An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Vir.277: 439–449.
- Dames, S., Pattison, D. C., Bromley, L. K., Wittwer, C. T., and Voelkerding, K. V. (2007): Unlabeled probes for the detection and typing of herpes simplex virus. Clinical Chemistry, 53:1847–1854.
- Davies, F. G. A. and Otema, G. (1981): Relationships of capripox viruses found in Kenya with two Middle Eastern strains and some Orthopox viruses. Research in Veterinary Science, 31: 253–255.
- Davies, F. G., Krauss, H., Lund, L. J., and Taylor, M. (1971): The laboratory diagnosis of lumpy skin disease. Research in Veterinary Science, 12:123-127.
- Davies, F.G, (1991): Lumpy skin disease, an African capripox virus disease of cattle. Vet. J., 147:489-502.
- Eeva, S.M. T., Caroline. R., Katarzyna, B.B., Nick, J. K., Shadi, A., Lorraine, F., Mark, R. H., Charles, E. L., and Peter, P.C. M. (2014): Characterization of sheep pox virus vaccine for cattle against lumpy skin disease virus. Antiviral Research 109 (2014) 1–6.
- El-Kholy, A.A., Soliman, H.M.T., and Abdelrahaman, K.A. (2008): Polymerase chain reaction for rapid diagnosis of a recent lumpy skin disease virus incursion to Egypt. Arab Journal Biotechnolgy, 11: 293-302.
- FAOSTAT, (2007): Database (http://faostat.fao.org). Rome. Accessed July 2011.
- Fenner, F., Bachmann, P. A., Gibbs, E. P. J., Murphy, F. A., Studdert, M. J., et al., (1987): Poxviridae. Veterinary Virology. New York, London Sydney, Tokyo, Toronto: academic press.Pp. 387-405.
- Gari, G, (2011): Epidemiological Study of Lumpy Skin Disease and Its Economic Impact in Ethiopia, Prevntive Veterinary Medicin, 11-161.
- Gari, G., Grosbois, V., Waret-Szkuta, A., Babiuk, S., Jacquiet, P., and Roger, F. (2012): Lumpy skin disease in Ethiopia: seroprevalence study across different agroclimate zones. Acta trop., 123, 101–106.
- Gari, G., Waret-szkuta, A., Grosbois, V., Jacquiet, P., and Roger, F. (2010): Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiology and Infection, 138:1657–1666.
- Gelaye, E., Belay, A., Ayelet, G., Jenberie, S., Yami, M., Loitsch, A., Tuppurainen, E., Grabherr, R., Diallo, A., and Lamien, C.E. (2015). Capripox disease in Ethiopia: Genetic differences between field isolates and vaccine strain, and implications for vaccination failure. Antiviral Res, 119: 28-35.
- Girma, Z. (2015): Isolation and molecular characterization of LSDV and vaccine effectiveness study in selected dairy farms of central Ethiopia. Msc thesis, College of veterinary medicine and agriculture, Addis Ababa University, Bishoftu, Ethiopia.
- Greth, A., Gourreau, J.M., Vassart, M., Nguyen-Ba-VyWyers, M., et al., (1992): Capripoxvirus disease in an Arabian Oryx (Oryx leucoryx) from Saudi Arabia. Journal. Wildlife Disease, 28: 295-300.
- Grooms, D. (2005): Raising Awareness about Lumpy Skin Disease Department of Large Animal Clinical Sciences. Michigan Dairy Review, 48: 824-1225.
- Gulbahar, M.Y., Davis, W.C., Yuksel, H., and Cabalar. M. (2006): Immunohistochemical evaluation of inflammatory infiltrate in the skin and lung of lambs naturally infected with sheep pox virus. Veterinary Pathology, 43: 67–75.
- HAIG, D.A. (1957): Lumpy skin disease. Bulletin Epizootic Disease of Africa, 5:421-430.
- Haines., and Chelack, B.J. (1991): Technical consideration for developing enzyme immune-histochemical staining procedures on formalin-fixed paraffin-embeded tissues for diagnostic pathology. Journal of veterinary Diagnostic investigation 3:101-112.
- Hedger, R.S., and Hamblin, C. (1983): Neutralizing antibodies to lumpy skin disease virus in African wildlife. Comprehensive Immunolology and Microbial Infectious Disease.6, 209–213.
- Industries Ministerial Council (available at http://www.oie.int/ file admin/ Animal Health in the World/docs/pdf/LSD 12 FINAL_27Jan09_. pdf).
- Ireland, D. C. and Binepal Y. S. (1998): Improved detection of capripoxvirus in biopsy samples by PCR. Journal of Virological Methods, 74: 1–7.
- Irons, P. C., Tuppurainen, E. S. M., and Venter, E. H. (2005): Excretion of lumpy skin disease virus in bull semen. Theriogenology, 63(5): 1290-1297.
- Kara, P. D., Afonso, C. L., Wallace, D. B., Kutish, G. F., Abolnik, C., et al., (2003): Comparative sequence analysis of the South African Vaccine strain and two virulent field isolates of Lumpy Skin Disease Virus. Archives of Virology, 148: 1335-1356.
- Khalafalla, A.I., GaffarElamin, M. A., and Abbas, Z. (1993): Lumpy skin disease: observations on the recent outbreaks of the disease in the Sudan. Rev Elev Med Vet Pays Trop.; 46(4): 548-550.
- Kitching, P. (2003): Vaccines for lumpy skin disease, sheep pox and goat pox. Vaccines for OIE list A and emerging animal diseases. Proceedings of a Symposium, Ames, IA, USA. Pp: 161–167.
- Kitching, R.P. and Smale, C. (1986): Comparison of the external dimensions of capripoxvirus isolates. Research in Veterinary Science, 41: 425–427.
- Kitching, R.P., Hammond, J., and Taylor, W. (1987): A single vaccine for the control of Capripox infection in sheep and goats. Reasearcc in Veterinary Science. 42: 53-60.
- Kivaria, F.M., Ruheta, M.R., Mkonyi, P.A., and Malamsha, P.C. (2007): Epidemiological aspects and economic impact of bovine Theileriosis (East Coast Fever) and its control: Preliminary assessment with special reference to Kibaha district, Tanzania, Vet. J., 173: 384-390.
- Lalani, A. S., Masters, J., Zeng, W., Barret, J., Pannu, R., et al., (1999): Use of Chemokine Receptors by Poxviruses. Science, 268: 1968-1971.
- Lamien, C.E., Le Goff, C., Silber, R., Wallace, D.B., Gulya, Z V., Tuppurainen, E., Madani, H., Caufour, P., Adam, T., EI, H.M., Luckins, A.G., Albina, E., and Diallo, A. (2011): Use of the Capri poxvirus homologue of Vaccinia virus 30 KDa RNA
- Le Goff, C., Lamien, C. E., Fakhfafh, E., Chadeyras, A., Abu-Adulugbad, E., et al (2009): Capripoxvirus G-protein-coupled chemokine receptor, a host-range gene suitable for virus-animal origin discrimination. Journal of General Virology, 90: 67–77.
- Lefèvre, P. C. and Gourreau, J. M. (2010): Lumpy Skin disease. In: Lefèvre, P.C., Blancou, J., Chalmette, R., Uilenberg, G. (Eds.), Infectious and Parasitic diseases of Livestock. Lavoisier, Paris. Pp: 393-407.
- Liew, M., Pryor, R., Palais, R., Meadows, C., Erali, M., et al. (2004): Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clinical Chemistry, 50: 1156–1164.
- MacDonald, R.A.S. (1931): Pseudo-urticaria of cattle.Annual Report for 1930. Department of Animal Health, Northern Rhodesia, 20–21.
- Magori-Cohen, R., Louzoun, Y., Herziger, Y., Oron, E., AraziA., Tuppurainen, E., Shpige, N.Y., and Klement, E. (2012). Mathematical modeling and evaluation of the different routes of transmission of lumpy skin disease virus. Veterinary Research, 43(1): 1297-1299.
- Mangana-Vougiouka, O.P., Markoulatos, G., Nomikou, K., Bakandritsos, N., and Papadopoulos, P. (2000): Sheep poxvirus identification from clinical specimens by PCR, cell culture, Immunofluorescence and agar gel immunoprecipitation assay. Molecular Cell Probes. 14: -310-305
- Matthews, R. E. F., (1982): Classification and nomenclature of viruses. Intervirology, 17: 1-99.
- Mebratu, G., Kassa, B., Fikre, Y., and Berhanu, B. (1984): Observations on the outbreak of lumpy skin disease in Ethiopia. Veterinary Tropicaux, 37:395–399.
- Moss, B., (1996a): Poxviridae: The Viruses and Their Replication. Fields Virology. 3rd Edition Lippincott-Raven Publishers, Philadelphia.Chapter. 83, Pp 2637-2671.
- Murphy, F. A., Gibbs, E. P. J., Horzinek, M. C., and Studdert, M. J. (1999): Veterinary Virology. Poxviridae. Pp 277-292
- OIE, (2009): World Animal Health Information Database - Version: 1.4. World Animal Health Information Database. Paris, France: World Or ganisation for Animal Health. http://www.oie.int/
- OIE, (2010): Lumpy skin disease. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Office International des Epizooties, World Organization for Animal Health, Paris. Pp: 1-13.
- Prozesky, L. and Barnard, B. (1982): A study of the pathology of lumpy skin disease in cattle. Onderstepoort. Journal of Veterinary Research, 49: 167-175.
- Radostits, M., Gay, C., Hinchcliff, W., and Constable, D. (2006): veterinary medicine A text book of the diseases of cattle, horses, sheep, pigs and goats 10th ed. WB Saunders Co., Philadelphia, USA. Pp: 1424- 1426.
- Radostits, M., Gay, C., Hinchcliff, W., and Constable, D. (2007): Veterinary medicine: a textbook of the disease of cattle. Horses, sheep, pigs and goats. 10th Ed. Sanders Ltd. Philadelphia. USA.1424-1426.
- Rashid, M. and Shank, R. (1994): United Nations development program emergencies unit for Ethiopia technical report: rough guide to animal diseases in Ethiopia.
- Rich, K.M., and Perry, B.D. (2011): The economic and poverty impacts of animal diseases in developing countries: new roles, new demands for economics and epidemiology. Preventive Veterinary Medicine. 101(3– 4):133–147.
- Rossiter, P. B., and Hammadi, N. L. (2009): Living with transboundary animal diseases (TADs). Tropical Animal Health and Production, 41, 999–1004.
- Rweyemamu, M., Paskin, R., Benkirane, A., Martin, V., Roe- der, P., and Wojciechowski K. (2000): Emerging diseases of Africa and the Middle East. In: House, J.A., K.M. Kocan, and E.P.J. Gibbs (eds), Tropical Veterinary Diseases – Control and Prevention in the Context of the New World Order, pp. 6170. Annals of New York Academy of Sciences, New York.
- Salib, F.A., and Osman, A.H (2011). Incidence of lumpy skin disease among Egyptian cattle in Giza Governorate, Egypt. Veterinary World 4(4):162-167.
- Shimshony, A., and Economides, P. (2006): Disease prevention and preparedness for animal health emergencies in the Middle East. Review Science and Technology Office International Epizoot. 25: 253– 269.
- Singh, M. K.P., Hussain, M.H., Rawahi, A. Al., Maawali, M. Al., Lamki, K. Al., et al. ,( 2007): Clinico-Histopathological findings and PCR based diagnosis of lumpy skin disease in the Sultanate of Oman. Pakistan Veterinary Journal, 32(2): 206-210.
- Somasundaram, M.K. (2011): An outbreak of lumpy skin disease in a holstein dairy herd in Oman: a clinical report. Asian Journal Animal Veterinary Advance. 2011: 6:851–859.
- Tamam, S.M. (2006): Isolation of Lumpy skin disease virus form naturally infected cattle previously vaccinated with live attenuated sheep poxvirus vaccine BS. Vet. Med. J., 16(1): 27-31.
- Tulman, E. R., Afonso, C.L., Lu Z., Zsak, L., and Kutish, G.F. (2001): Genome of Lumpy Skin Disease Virus. Journal of Virology, 75(15): 7122-7130.
- Tulman, E., Afimo, C., Lu, Z., Zsak, L., Sur, J., and Sandybaev, N. (2002): The genomes of sheep pox and goat poxviruses. Journal Virology, 76(12): 6054–61.
- Tuppurainen, E. S. M., and Oura, C. A. L. (2011): Review: Lumpy Skin Disease: An Emerging Threat to Europe, the Middle East and Asia, Institute for Animal Health, Pirbright, Surrey, UK.
- Tuppurainen, E., Venter, E., and Coetzer, J.A.W. (2005): The detection of Lumpy Skin Disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort Journal of Veterinary Research, 72: 153-164.
- Tuppurainen, E.S.M., and Oura, C.A.L. (2012): Review: Lumpy skin disease: An Emerging Threat to Europe, the Middle East and Asia. Transboundary and Emerging Diseases; 59:40– 48.
- Tuppurainen, E.S.M., Lubinga, J.C., Stoltsz, W.H., Troskie, M., Carpenter, S.T., et al.(2013a) Mechanical transmission of lumpy skin disease virus by Rhipicephalus appendiculatus male ticks. Epidemiology and infection, Epidemiology Infection. 141 (2): 425-430.
- Vorster, H., and Mapham, H. (2008): pathology of lumpy skin disease. Livestock Health and Production Review, 1:16-21
- Weiss, K.E. (1968): Lumpy skin disease virus. IN Virology Monographs, Volume 3, Pp: 111-131. Vienna, New York, Springer Verlag.
- Wittwer, C. T., Reed, G. H., Gundry, C. N., Vandersteen, J. G. (2003): High resolution genotyping by amplicon melting analysis using LC Green. Clinical Chemistry, 49: 853–860.
- Woodroofe, G. M., and Fenner, F. (1962): Serological relationship within the poxvirus group: an antigen common to all members of the group. Virology, 16: 334-341.
- Woods, J.A. (1988): Lumpy skin disease-A review. Tropical Animal Health Production, Department of Federal Veterinary Research. 20:11 17.
- Wu, Yuan, H., Zhang, X., Liu, W., Xu, J., et al. (2011): Development and inter laboratory validation of unlabeled probe melting curve analysis for detection of JAK2 V617F mutation in polycythemia vera. PLoS One 6: e26534.
- Yeruham, I., Perl, S., Nyska, A., Abraham, A., Davidson, M., Haymovitch, M., Zamir, O., and Grinstein, H. (1994): Adverse reactions in cattle to a capripox vaccine. Vet. Rec. 135: 330–332.
- Young, E., Basson, P.A., and Weiss, K. E. (1970): Experimental infection of game animals with lumpy skin disease virus (prototype strain, Neethling). Onderstepoort Journal of Veterinary Research, 37: 79-88.