Information
Journal Policies
ARC Journal of Animal and Veterinary Sciences
Volume-2 Issue-1, 2016
Abstract
This study aims to find out the differences between sexes on basis of craniometric measurements obtained from 3-dimensional (3D) reconstruction of multidetector computed tomography (MDCT) images in New Zealand rabbits. For this purpose, 12 adult healthy New Zealand rabbits of both sexes were used as materials. After MDCT images of the skulls were taken, they evaluated in a personel computer and reconstructed 3-dimensionally with the 3D translator component of the (Mimics) computer. A set of craniometric measurements over these reconstruction images is taken to bring out some skull indexes. This study revealed that that there were statistical differences (P < 0.05) among sex related-measurements such as skull length, nasal length, cranial length, skull width, nasal width, cranial width and skull index. Moreover, the rates were compared with other studies in the literature and were brought out the morphologic diversities among species. Specially, it has been thought that the skull indexes recorded in this work can shed light on further craniometric studies.
2.KEYWORDS
3. INTRODUCTION
4.MATERIALS AND METHODS
5.RESULTS
6.DISCUSSION
7.ACKNOWLEDGEMENT
8.REFERENCES
AUTHOR DETAILS
Rania Abdel Rahman Elgawish1, Heba M. A. Abdelrazek2, Mohamed Ghanem3
1 Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
[email protected]
2 Department of Physiology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
[email protected]
3 Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
[email protected]
Keywords
Postmortem, rats, electrolytes, adrenal gland
INTRODUCTION
The importance of determining the time after death is crucial to forensic cases. Postmortem changes include different processes such as body cooling, hypostasis, rigor mortis, metabolite concentrations, enzyme activity and morphological changes [1, 2]. Most of the chemical methods used for assessment the time after death are based on some parameters, one of the parameter is autolysis, with rupture of cell membranes [3]. There are few scientific approaches based on chemical evaluation that can be used to estimate the postmortem interval (PMI). Changes in blood and other tissues fluid biochemistry are assessed; however considerable inaccuracy has been determined. The most used chemical indicator of the PMI before putrefaction is the potassium content of the vitreous humor [4]. The higher the concentration, the more suitable is the substance for the estimation of the PMI [5].
The adrenal gland is one of the first autolysed tissues in the body. There are studies that describe the relationship between the postmortem interval and autolysis of the myocardium and sublingual gland. However, postmortem interval studies that include the adrenal gland are lacking [6]. The volume of the adrenal gland increased over time after death [6]. The loss of adrenocortical lipids can be detected after a long time [7], and the nuclei of adrenal cells decreased in size, followed by cellular shrinkage [8].
Historically, analysis of postmortem blood was the most extensively studied because blood carries most substances present in a body [4], although vitreous humor is being currently examined to determine PMI. This is mainly due to the fact that vitreous humor is well protected and isolated from the blood stream and microbes. Thus, the autolytic changes proceed more slowly compared with blood. This experimental study was designed to determine the postmortem changes in electrolytes and some ions in vitreous humor of adult male albino rats as well as lactate levels in skeletal and cardiac muscles. Moreover, to investigate the relationship between the postmortem interval and autolytic changes in the adrenal gland of male albino rats using special staining method.
MATERIALS AND METHODS
Nine, three-month old male albino rats, weighing 185 - 225 g, were fed ad libitum and were kept at Laboratory Animal House of Faculty of Veterinary Medicine, Suez Canal University, Egypt. Rats underwent cervical dislocation. Three of the rats under cervical dislocation were separated for immediate dissection. These rats were used for determining postmortem “0 h values” the remaining 6 rats were divided into two groups (3 rats / group) to investigate postmortem “6 h values” and postmortem “12 h values”. The rats were kept at constant temperature (14 ± 2 ºC) throughout the time intervals of the experiment. All the protocols regarding the study were approved by institutional ethical committee and conducted according to the ethical guidelines for the use of animals in laboratory experiments of the Faculty of Veterinary Medicine, Suez Canal University, Egypt.
For each time interval postmortem, the vitreous humor was gently withdrawn by syringe, and each sample was placed in a polypropylene tube. The samples were stored at -18 °C until evaluation of sodium (Na), chloride (Cl) and potassium (K) electrolytes levels as well as calcium (Ca) and phosphorus (P) ions.
Electrolytes (Na, Cl and K) as well as ions (Ca and P) were analyzed on Roche Cobas 6000 auto analyzer (Roche Diagnostics, Mannheim, Germany) with the original reagents (Roche Diagnostics GmbH D- 68298 Mannheim, Germany) at the Laboratory of Clinical Pathology, Faculty of Medicine, Suez Canal University.
At each time interval (0, 6 and 12 h) postmortem, the rats that were set aside following cervical dislocation underwent immediate dissection. The muscle of the right thigh and the heart muscle were put in to 10 ml polypropylene tubes, and kept at -20 ºC until lactate levels were analyzed.
At the time of sample preparations for lactate analysis, 0.5 g of the skeletal and cardiac muscles was taken at 0, 6, and 12 h following cervical dislocation. Samples were put on 2.5 ml of phosphate buffer saline (pH 7.2) to prepare tissue homogenate. The sample were centrifuged at 3000 rpm for 15 min. and sent for lactate measurements analysis on Roche Cobas 6000 auto analyzer (Roche Diagnostics, Mannheim, Germany) at the Laboratory of Clinical Pathology, Faculty of Medicine, Suez Canal University.
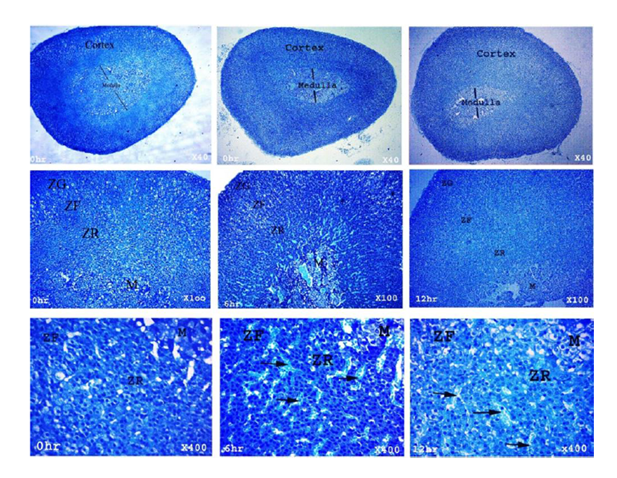
The left adrenal gland was excised and fixed in 10% formalin solution and then embedded in paraffin according to standard procedures. Ten to fifteen sections of 30 μm thick were obtained from each left adrenal gland and stained with hematoxylin eosin (H&E). Other sections were stained with Diff-Quick stain (Sysmex, Kobe, Japan) as a special stain of adrenal gland medulla. The volume of the cortex and medulla of the adrenal gland was evaluated using Image Analysis software (Visual measure 32 for Windows, Version 1.7, Rise Corporation, Japan) at 0, 6, and 12 h postmortem.
All values were expressed as mean ± standard error of the mean. Comparisons of electrolytes, ions, lactate level and relative weight of the adrenal gland obtained at 0, 6 and 12 h postmortem were performed with one way analysis of variance (ANOVA). Inter-group comparisons were made with Tukey's multiple comparison tests. The volume of the cortex, medulla and total volume of the adrenal gland were evaluated with Kruskal-Wallis test. When a significant difference was detected, the Dunns test was used to identify the source of the difference. A P value of < 0.05 was considered to indicate significance. All the analysis was done using GraphPad Prism (Version 5.01, GraphPad Software, San Diego, USA).
RESULTS
The levels of Na and Cl showed non-significant (P=0.4) difference at 0, 6 and 12 h postmortem, in spite of the slight decrease in the Na and Cl values by the time interval from 0 to 12 h after death of the rats. The K levels was increased significantly (P=0.01) in rats at 0 h compared to the levels at 12 h postmortem. The Ca levels was decreased significantly (P=0.02) in rats at 0 h compared to that levels at 12 h postmortem. In contrary, the P values was not significantly (P=0.2) changed in rats at 0, 6 and 12 h postmortem (Table 1).
The lactate levels in skeletal muscle of the rats at 12 h postmortem showed significant (P=0.04) increase than that levels at 0 h. Moreover, the lactate levels in cardiac muscle was significantly (P=0.001) increased at 6 and 12 h compared to 0 h postmortem (Table 1).
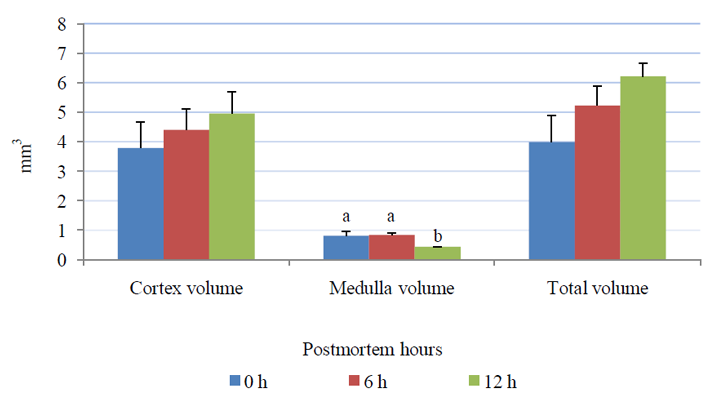
The relative weight of the left adrenal gland (0.013±0.001) g at 12 h was significantly higher (P= 0.04), than that at (0.007±0.001) g. Total volume of the adrenal gland has been increased from (3.98±0.91) mm3 at 0 h to (6.21±0.44) mm3 at 12 h postmortem without reaching a significant difference (P= 0.14). The cortex of the adrenal gland increased from (3.79±0.88) mm3 at 0 h to (4.96±0.73) mm3 at 12 h postmortem without reaching a significant difference (P= 0.53), however, a significant decrease in medullar volume (P= 0.03) was detected between 0 h (0.80±0.16) mm3 and 12 h (0.43±0.01) mm3 (Fig. 1).
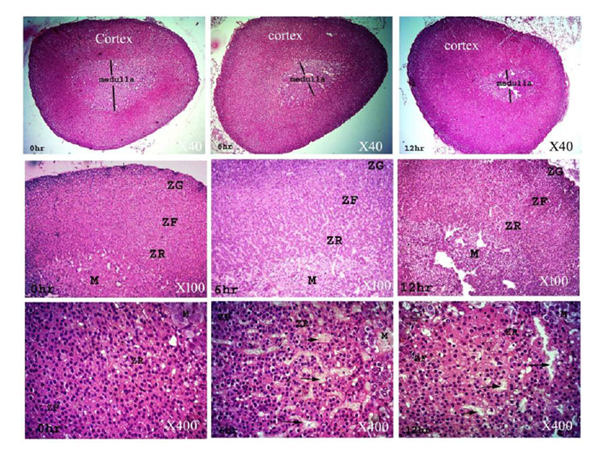
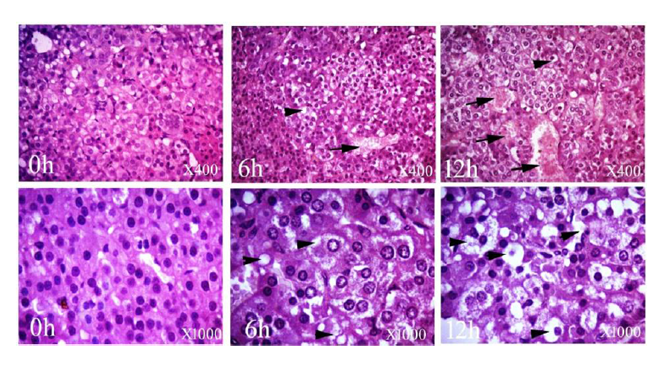
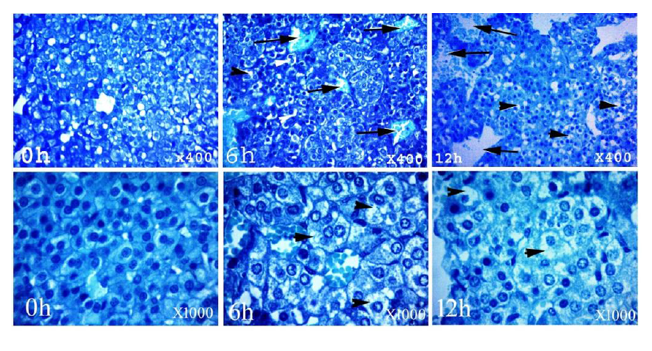
The adrenal gland at 0 h had normal maintained architecture with well differentiated cortex and medulla. The cortex showed normal histological structure of the three zones. Zona granulosa (ZG) showed normal epithelium with intact basement membrane and most of the nuclei were completely hyperchromatic with rarely pyknotic or vesicular nuclei. Zona fasciculata (ZF) and zona reticularis (ZR) showed normal non dilated sinusoids with prominent endothelial cells. Most cells had hyperchromatic and eosinophilc abundant cytoplasm. Medulla (M) was distinct and well differentiated from cortex; most of the nuclei were completely hyperchromatic with normal intact blood vessels. At 6 h postmortem, architecture was slightly maintained and the cortex could be differentiated from the medulla. The cortex was expanded in expense of medulla, while at 12 h a marked expansion was observed. Cellular outline of ZG, ZF and ZR was not clear and cytoplasm showed vacuolation. Most of the nuclei were completely pyknotic and vesicular nuclei were seen at 6 and 12 h. The sinusoids showed moderate to marked dilatation with few prominent endothelial cells at 12 h. The cellular outline of the medulla was indistinct. Pyknotic nuclei were observed in several places at 12 h (Fig. 2-5).
DISCUSSION
Biochemical profiles from body fluids taken after death can provide useful information regarding cause of death and have the potential to provide PMI estimations if suitable postmortem biochemical markers are identified [9]. In the present study, the levels of Na and Cl showed non-significant (P=0.4) difference at 0, 6 and 12 h postmortem, in spite of the slight decrease in the Na and Cl values by the time interval from 0 to 12 h. Concentrations of sodium and chloride decreased by 1 mmol/L per hour for the first 3-52 hours postmortem in serum [4, 10-13]. These two markers are not reliable enough to be used as a PMI indicator; they are now measured only from the vitreous humor, rather than serum [4, 10]. In the current experiment, K level was increased significantly (P=0.01) in rats at 0 h compared to the levels at 12 h postmortem. Serum K levels rapidly increased over the first 1-2 h after death [11, 14, 15]. As with Na and Cl, K is measured in the vitreous humor. This is because it has been repeatedly shown to rise rapidly in the first 6-12 h after death and then linearly after 24 h [4, 10, 13, 15]. In general the discrepancy in the results of postmortem electrolytes values among researchers might be due to temperature, sampling techniques, differences between each eye as well as the age of the individual [16].
In the present study, Ca levels was decreased significantly (P=0.02) in rats at 0 h compared to that levels at 12 h postmortem. In contrary to our study, Jetter [14] reported that the plasma calcium concentration was relatively stable from 12-24 h postmortem. Hodgkinson and Hambleton [17] found that plasma calcium concentration increased rapidly within an hour postmortem from 4 mmol/L to reach 9.4 mmol/L after 5 h before decreasing back to antemortem levels by 11 h. Fekete and Brunsdon [18] found a wide range of serum calcium concentrations within 2-36 h postmortem. All of these researchers used different methods to analyze serum calcium concentrations and that may explain why the findings are so variable, indicating that blood calcium levels are not a reliable indicator of PMI. In the current work, P values was not significantly (P=0.2) changed in rats at 0, 6 and 12 h postmortem. In contrary to our study, postmortem increase was observed for organic and inorganic phosphorus [14]. Inorganic phosphate was reported to be present at antemortem concentrations of 0.6–0.9 mmol/L and rises within one hour postmortem to reach concentrations of 6.6 mmol/L by 18 h after death [14].
Autolysis starts approximately 4 min after death. As cells of the body are deprived of oxygen, carbon dioxide in the blood increases, pH decreases, and wastes accumulate, which poison the cells. In this study, skeletal muscle tissue was selected for analysis because the skeletal muscle tissue autolytic changes and putrefaction proceed slower compared to body fluids and organs [3]. In the present work, the lactate levels in skeletal muscle at 12 h postmortem showed significant (P=0.04) increase than that levels at 0 h. Moreover, the lactate levels in cardiac muscle was significantly (P=0.001) increased at 6 and 12 h compared to that at 0 h postmortem. Postmortem serum lactate in human heart blood has been increased in the blood 20 folds one hour after death and rose 50-70 times higher than antemortem levels by 24 h [14]. Lactate has also been measured postmortem in cerebrospinal fluid [13], vitreous humor [19]. It has been suggested that lactate concentrations in the bloodstream postmortem may be affected by lactate diffusion from muscular tissues and because of blood glycolysis in the vessels, which would make lactic acid an unsatisfactory marker for PMI [13]. However, further research is required to determine if lactate is a possible marker for estimation of PMI.
In this study, the relative weight of the adrenal gland has been increased at 12 h postmortem. Nery et al. [20] determined a 34.3% increase in the glandular mass of the sublingual gland from 0 to 24 h and reported that this increase is related to transition of liquids originating from the surrounding tissues. In the present study, total volume of the adrenal gland as well as adrenal cortex has been increased, however, a significant decrease in medullary volume (P= 0.03) was detected at 12 h. Marked expansion of cortex was detected. The cellular outline of the medulla was indistinct. Pyknotic nuclei were observed in several places at 12 h. In accordance to our findings, Kurtulus et al. [6] observed that an enlargement of the total cell size of adrenal gland due to widespread intracytoplasmic vacuolar degenerative change as well as nuclear changes, such as karyolysis, pyknosis and karyorrhexis. The first symptom of the cellular damage is mostly cellular swelling, which is known as ""hydropic change"" or ""vacuolar degeneration"". Additionally, Kurtulus et al. [6] observed a substantial amount of edema in the intercellular space of both adrenal cortex and adrenal medulla. Additionally, in the current study, significant decrease in the medulla was observed in the medulla volume in parallel to the time elapsed after death. Alejandro and Strafuss [8] previously reported decrease in the adrenal gland medullar cell nuclei sizes with pyknosis, cytoplasmic vacuolization, cellular shrinkage, karyolysis and cell dissociation in the postmortem period. In conclusion, K and Ca in vitreous humor and lactate levels in skeletal and cardiac muscles were changed by postmortem time intervals. Histopathological changes and the volume of adrenal gland could be used reliably in determining the postmortem intervals. A more detailed study including large number of rats is planned in the near future.
ACKNOWLEDGEMENT
The authors wish to thank Prof. Dr. Amina Dosuky and Dr. Doaa Hosny at Faculty of Veterinary Medicine, Suez Canal University for their useful cooperation during the study.
REFERENCES
- Knight B., Forensic Pathology. 2nd ed. London, Arnold, pp. 79-80 (1996).
- Henssge C., Madea B., Estimation of the time since death. Forensic Sci Int, 165, pp. 182-184 (2007).
- Dogan K.H., Gunaydin G., Demirci S., Koc S., Postmortem changes in element levels in rat skeletal muscle tissue. Turkiye Klinikleri J Med Sci, 30, pp. 1332-1338 (2010).
- Coe J.I., Postmortem chemistry update. Emphasis on forensic application. Am J Forensic Med Path, 14, pp. 91-117 (1993).
- Madea B., Is there recent progress in the estimation of the postmortem interval by means of thanatochemistry? Forensic Sci Int, 151, pp. 139-49 (2005).
- Kurtulus A., Acar K., Sorkun H., Kelten C., Boz B., The relationship between adrenal gland morphometric changes and postmortem interval in rats: A stereological study. Legal Med, 14, pp. 214-218 (2012).
- Reinhard B.D., Forensic histopathology: fundamentals and perspectives, 1st edn. Springer, Berlin (2012).
- Alejandro V.S., Strafuss A.C., Microscopic postmortem changes in adrenal glands of the domestic fowl. Avian Dis, 28, pp. 374-85 (1984).
- Lundquist F., Methods of forensic science. London, New York: Interscience Publishers (1963).
- Spitz W.U., ed. Spitz and Fisher's medicolegal investigation of death. Guidelines for the application of pathology to crime investigation. 4th ed. Illinois: Charles Thomas Publisher (2006).
- Coe J.I., Postmortem chemistries on blood with particular reference to urea-nitrogen, electrolytes and bilirubin. J Forensic Sci, 19, pp. 33-42 (1974).
- Singh D., Prashad R., Parkash C., Bansal Y.S., Sharma S.K., Pandey A.N., Linearization of the relationship between serum sodium, potassium concentration, their ratio and time since death in Chandigarh zone of north-west India. Forensic Sci Int, 130, pp. 1-7 (2002).
- Schleyer F., Determination of the time of death in the early postmortem interval. Methods Forensic Sci, 2, pp. 253-293 (1963).
- Jetter W., Postmortem biochemical changes. J Forensic Sci, 4, pp. 330-341 (1959).
- Coe J.I., Vitreous potassium as a measure of the postmortem interval: an historical review and critical evaluation. Forensic Sci Int, 42, pp. 201-213 (1989).
- Madea B., Henssge C., Hönig W., Gerbracht A., References for determining the time of death by potassium in vitreous humor. Forensic Sci Int, 40, pp. 231-243 (1989).
- Hodgkinson A., Hambleton J., Elevation of serum calcium concentration and changes in other blood parameters after death. J Surg Res, 9, pp. 567-574 (1969).
- Fekete J.F., Brunsdon D.F.V., The use of routine laboratory tests in postmortem examinations. Can Soc Forensic Sci J, 7, pp. 238-254 (1974).
- Jaffe F.A., Chemical postmortem changes in the intraocular fluid. J Forensic Sci, 7, pp. 231-237 (1962).
- Nery L.R., Moreira C.R., Cestari T.M., Taga R., Damante J.H., Postmortem acinar autolysis in rat sublingual gland: a morphometric study. J Appl Oral Sci, 18, pp. 509-514 (2010).