Information
Journal Policies
Ultrasound Guided Intra-Articular Pulsed Radiofrequency in Patients with Primary Chronic Knee Osteoarthritis
Omnia Ahmed Fathy Ibrahim Aly1*, Sahar Ahmed El- Karadawy2, Abd El-Latif Ahmed Gab Allah3, Noha Abd El-Halim El-Sawy3, Sarah Sayed El-Tawab3
2.Department of Anesthesia and Pain Management, Medical Research Institute, Alexandria university, Alexandria, Egypt.
3.Department of Physical Medicine, Rheumatology and Rehabilitation, Faculty of medicine, Alexandria university, Alexandria, Egypt.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Osteoarthritis (OA) is a progressive disabling joint disease manifested by pain and limited function. Peripheral and central sensitization are contributors in OA pain. The goals of treatment in OA are pain control, function improvement and disability reduction.
Purpose: To study the effect of intra-articular (IA) Pulsed radiofrequency (PRF) guided with ultra-sound (US) on chronic pain in patients with primary knee OA.
Participants and Methods: Fifty-two patients grade 2 and 3 Kellgren-Lawrence classification were assessed for eligibility. The intervention included two stages. One was a diagnostic block using lidocaine 2% ,4 milliliters (ml). Two used PRF only if OA pain was relieved by more than 50% after the first stage block. The study outcomes were measured using Numerical rating scale (NRS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Score.
Results: Intra-articular knee injection using PRF led to significant pain reduction and functional improvement in patients with primary chronic knee OA pain. There were minimal post-intervention adverse events.
Conclusion: PRF is an effective treatment for OA patients who did not achieve pain relief by conservative therapy, not fit for surgery or refuse surgery. Further trials with larger sample size and longer follow-up are recommended.
Ultrasound; Guided; Intra-articular; Pulsed radiofrequency; Chronic; Knee osteoarthritis, Anesthesiology
Osteoarthritis (OA) is a chronic disease which has a major impact on patients' health through pain and disability. The estimated prevalence of OA in the general population is 8-15% making it the most common joint disease[1]. Knee OA incidence increases by age and further with longer lifetime and higher average weight of the population[2]. Symptoms can vary greatly among patients including joint pain and stiffness, swelling, decreased function, and cracking or grinding noise with joint movement[3].
The exact mechanism of pain in OA is not well known. It may be attributed to peripheral and central sensitization, where the pro- inflammatory mediators interleukin 6 (IL-6) and tumor necrosis factorα (TNF-α) not only promote inflammation but also sensitize mechanosensitive neurons and awaken silent nociceptors in the affected joint[4]. Activation of intracellular kinases as a result of receptors activation controls a secondary message cascade and causes genetic modulation. Pro-inflammatory mediators also contribute in the generation of mechanical hypersensitivity, hyperalgesia and allodynia associated with OA[4].
OA diagnosis can be done through following the American College of Rheumatology (ACR) criteria for the diagnosis of primary knee OA. The diagnosis is done through clinical, radiological and laboratory findings[5]. Knee OA can be managed conservatively or surgically. Conservative treatment include physical therapy, analgesics drugs, intra-articular (IA) injection of steroids, and visco-supplementation[6]. OA patients who did not achieve pain relief by conservative therapy, not fit for surgery or refuse surgery may have total knee joint replacement[7,8].
Pulsed radiofrequency (PRF) is a new modality used for management of pain of OA. It was introduced in 1998 as a nonlytic alternative to convention RF[9]. The tissue temperature does not exceed 42 C thus no irreversible tissue damage occurs[10]. PRF injection avoids thermal lesioning and exerts its effect through rapid changing electrical field.
PRF with its electrical field may modulate small pain information carrying C fibers with less effect on the larger fibers[11]. PRF appears to interrupt signals only in unmyelinated C fibers[12]. Another theory on PRF action is the neurobiological pathway. The electric field alters the pain-signaling process via increased expression of c-fos, a non-specific marker of cellular activity[13,14]. c-fos is not a specific nociceptive pathway marker and indicates only the activation of cells[15].
The electrical field effect on C fibers and the consequent modulation of pain also apply when the electrode is positioned intra-articularly in joints. In large joints, such as the knee and shoulder, the pain modulating effect of the electrical field in the joints is thought to be the local effect on the immune system[16,17]. Previous studies reported attenuation of the production of pro-inflammatory cytokines such as interleukin IL-1b, IL-6 and TNF-alpha after the application of PRF, exhibiting the anti-inflammatory effect of PRF[18]. In addition, there are hypotheses that suggested that PRF electrical field enhances intra-articular cartilage protective and regenerative mechanisms[18]. Ultrasound (US) guidance improves the success rate and safety of intra-articular invasive procedures. It is more superior than computed tomography and Fluoroscopic guided techniques, as it is a bed side tool, less expensive and carries no hazards of radiation[19,20].
Considering the insufficient evidence towards the role of IAPRF in the management of primary osteoarthritis related pain, the current study was designed to evaluate the effectiveness and the safety of US guided IA PRF on chronic pain associated primary knee osteoarthritis not responding to other conservative treatments.
2. Materials And Methods
The current study isopen-labeled, non-randomized, prospective and single-armed. After taking the approval of the ethical committee of the Faculty of Medicine, University of Alexandria and obtaining consent from each patient, 52 patients with primary knee OA, grades 2 or 3 Kellgren-Lawrence (KL) classification [21] who could not achieve satisfactory pain relief with conservative therapies (physical therapy, analgesic drugs (nonsteroidal anti-inflammatory, opioid), intra-articular injection of steroids or visco-supplementation) during the last 3 months, and refused or were unfit for surgery were recruited from the Outpatient Clinic of the Physical Medicine, Rheumatology and Rehabilitation Department, Faculty of Medicine, Alexandria University, Egypt starting from May 2017 till December 2017.Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)[22,23] Score and Numerical rating scale (NRS)[24] for pain were recorded for patient selection. A score of 5 or more on NRS was used for recruitment. The selected patients received IA knee injection in the block room of the Medical Research Institute hospital, Alexandria University. The injection was done in two stages under local anesthesia and conscious sedation, [10] using intravenous midazolam 2 milligrams (mg)and fentanyl 20 micrograms (mcg), and guided by ultrasound (Sonosite S-Nerve, USA) machine.
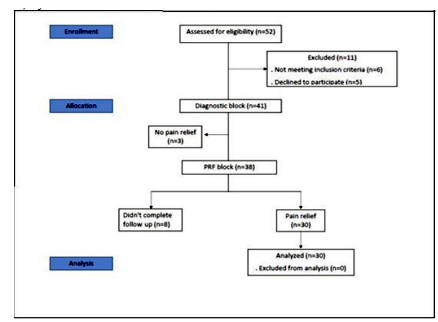
Fifty-two patients were assessed for eligibility in the current study, 11 were excluded and 41 were enrolled. Eight patients did not show up after their first follow up visit. Patients were excluded (six patients) if they had clinically assessed secondary knee OA, associated pain causes such as radicular pain, coagulation disorders, systemic or local infection in the interested area of injection, psychiatric disorders, excessive use of opioids, allergies to local anesthetics or cardiac pacemakers[25]. Some patients refused to participate in the study (five patients) and others did not achieve pain relief after the diagnostic block trial (three patients). Figure 1.
Demographic data collection, A complete history for knee OA, medical and drug history taking, clinical examination, radiological assessment of knee joints (done using plain X-Ray of both knees in antero-posterior and lateral views on standing position). The disease severity was assessed using the Kellgren - Lawrence grading scale (four grades for radiological disease severity). WOMAC Score (24 parameters, 0-4 point each one, three subscales; Pain [5 questions], Stiffness [2 questions] and difficulty in daily activities [17 questions], minimum score 0 [best score] and maximum score 96 [worst score]) and NRS for pain (0-10 point, 0 means no pain and 10 is the worst pain) were recorded pre-intervention (baseline evaluation) and at 1 ,4 and 12 weeks follow up post-intervention visits at the Outpatient Clinic of the Physical Medicine, Rheumatology and Rehabilitation Department, Faculty of Medicine, Alexandria University.
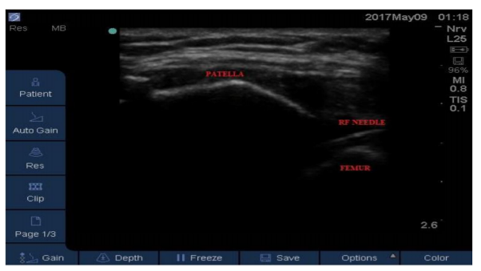
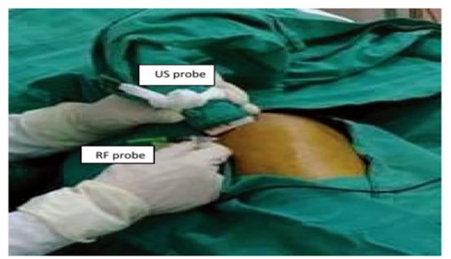
Patient was placed in a supine position with a pillow under the knee putting it in about 45 degrees of flexion. The knee joint skin was cleaned with alcohol and was wiped with an iodine-based antiseptic solution. The ultra-sound linear 6-13 mega-hertz (MHz) transducer was applied on the upper outer border of the patella in the transverse axis and sonoanatomy of the knee joint was reviewed. The superolateral joint space was identified and a short beveled 50 mm needle was advanced through an in-plane approach towards the center of the joint with an angle of 45 degrees to the skin. After proper visualization of the needle tip 4 ml of lidocaine 2% were injected intra-articularly as a diagnostic block. After a week, the patients who experienced a reduction of pain by at least 50% received US guided PRF block for the same knee. A 22 gauged 50 mm radiofrequency (RF) insulated curved cannula, with a 10 mm active tip was advanced toward the center of the joint. Then, PRF was applied with 42°C temperature and a pulse width of 20 milliseconds for 15 minutes[26-28]. Figure (2 and 3) Diagnostic block testing is not mandatory yet it confirms that the source of pain is the knee giving better prognostic value and higher chances of success with PRF injection [25,29].
Patient satisfaction with medical care and postoperative pain was evaluated by the patient satisfaction questionnaire which assessed the satisfaction with information and medical care before the patients were discharged[30]. It is 7 items, self-report scale. Each item was rated on a 0-3 point scale: 0 not satisfied and 3 highly satisfied[31]. Complications to IA injection as granulomatous inflammation of the synovium [32], aseptic acute arthritis [33,34] and septic arthritis[35,36] were not encountered. Minimal complications like ecchymosis or pain at the site of injection occurred.
Group sample size of 30 patients achieves 90 % power to detect a difference in NRS score of (1) in a design with 4 repeated measurements (pre- intervention, 1 week, 1 month and 3 months post-intervention), when the standard deviation is (1.5), and the alpha level is .05. The sample size was calculated using PASS program version 12.0.2. Thirty-eight patients with primary knee OA were enrolled in the study to raise significance of power[31,37,38].
3. Statistical Analysis
Statistical analysis[39] was performed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) [40]. Normality testing was done through the Kolmogrov-Smirnov test. Normally distributed quantitative variables were described by the mean and standard deviation, while not normally distributed data were described through the median and range. Qualitative variables were described by their frequencies and %. Analysis of variance (ANOVA) was used to compare repeated measures for comparison between different periods. P was considered statistically significant at ≤ 0.05.
4. Results
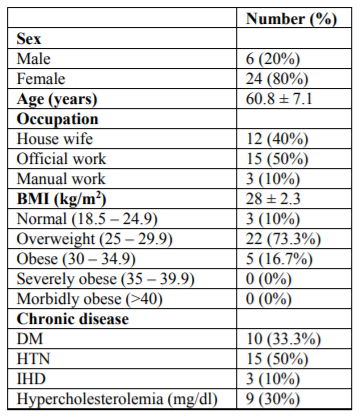
The studied group was distributed according to sex into 6 males (20% of the patients) and 24 females (80 % of the patients). The mean age was 60.8 ± 7.1. According to occupation, 12 patients were housewives, 15 were official workers and 3 were manual workers representing 40%, 50% and 10%, of the patients, respectively. Only 3(10%) patients had normal body mass index (BMI) (18.5 - 24.9 kg/m2), 22 (73%) patients were overweight (BMI =25 - 29.9 kg/m2) and 5(16%) patients were obese (BMI=30 - 34.9 kg/m2). Table (1)
Hypertension (HTN) was the major co-morbidity in our studied group, 15 patients had HTN representing 50% of the patients. Other co-morbidities were, Diabetes Millets (DM) (33.3%), Ischemic heart disease (IHD) (10%)and Hypercholesterolemia (30%). Table (1)
The mean value for patient satisfaction score was19.9± 1.9. The following scores were recorded pre-procedure and on week 1,4 and 12 post-procedure follow up visits
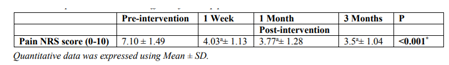
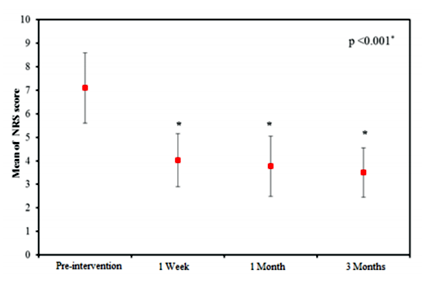
Table (2) and figure (4) show that there was significant reduction in NRS pain score (p = < 0.001*) over the follow-up period of 3 months in comparison to pre-intervention value. After achieving pain relief with PRF injection, three of the female patients started pool exercises in order to lose weight and strengthen the quadriceps muscle.
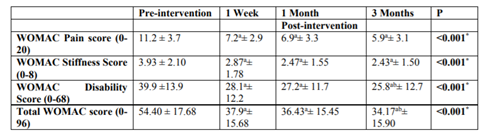
Table (3) shows that the pain score decreased significantly (p = < 0.001*) over the follow-up period of 3 months.
Table (3) shows that there was significant improvement in stiffness score (p = < 0.001*) over the follow-up period of 3 months.
Table (3) shows that there was significant improvement in disability score (p = < 0.001*) over the follow-up period of 3 months.
Table (3) shows that there was significant improvement in total WOMAC score (p = < 0.001*) over the follow-up period of 3 months.
Disability and total WOMAC scores at 12 weeks were significantly reduced when compared to 1-week scores (p = <0.001*).
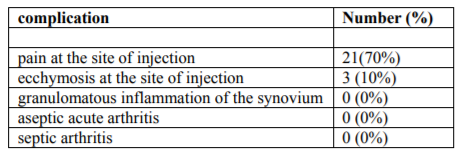
In the current study, 21 patients (70%) complained of post-intervention pain at the site of injection. 3 patients (10%) complained of ecchymosis at the site of injection post-intervention. No other complications were encountered. Table (4).
5. Discussion
The focus of the treatment of knee OA lies in relieving refractory pain, promoting the quality of life and improving knee function. Therefore, recent researches were directed towards, intra articular ozone injection,[41] platelets rich plasma injection[42] or intra articular neuro modulations[16].
The current study showed that pulsed radiofrequency guided by ultrasound was an effective line of treatment in knee osteoarthritis. As PRF resulted in significant improvement, not only in NRS pain score, but also in the WOMAC disability, pain and stiffness scores.
There was significant improvement in NRS pain score (p = < 0.001*) over the follow-up period of 3 months. After achieving pain relief with PRF injection, three of the female patients started pool exercises in order to lose weight and strengthen their quadriceps muscle. The pain relief the patients achieved encouraged them to pursue a healthier life style in order to attain that relief. It was noticed that study patients admitted to decreased analgesic consumption post-intervention.
Patients with OA experience short-lasting inactivity stiffness or gelling of joints[43]. The WOMAC stiffness score of the current study was significantly improved (p = < 0.001*) over the follow-up period of 3 months. Several studies have reported a relationship between elevated levels of pro-inflammatory cytokines and morning stiffness 'gelling phenomenon'[44,45]. The level of these cytokines was highly elevated in patients early in the morning. TNF and IL-6 are pro-inflammatory cytokines which accumulated during the night and might have been the trigger of morning stiffness when the patients woke up in the morning[46,47]. PRF had a role in decreasing the concentration of these pro-inflammatory cytokines[18] and this might explain the improvement in stiffness scores.
The WOMAC disability and total WOMAC scores at 12 weeks were significantly reduced when compared to 1-week scores (p = < 0.001*). It was noticed that when the patients felt pain relief post-intervention they disregarded the advice of avoiding excessive knee flexion more than 90 degrees and excessive exercise. They proudly reported on week 1 follow up visit how they can squat and climb stairs without much difficulty as before. They were advised that this would cause damage to their knees and decrease the period of pain relief provided by PRF. They began to follow orders strictly after that which might explain the significant difference in scores between week 1 and 12.
No major complications to IA knee injections as granulomatous inflammation of the synovium [32] , aseptic acute arthritis [33,34] and septic arthritis [35,36] occurred to the studied patients but rather some minimal complications like ecchymosis or pain at the site of injection[32]. This may be attributed to the accuracy given by US guided injection which was more accurate than landmark-guided injection [48]. US guided injection provided accurate needle tip positioning which reduced local complications[49].
Yan Yuan et al[50]. investigated the clinical effect of intra-articular PRF on the pain caused by refractory knee osteoarthritis. They compared IA dexameth as one injection to PRF. The study recorded the intra- articular concentrations of TNF-α, matrix metalloproteinase-3 (MMP-3) andIL-1in synovial fluid, VAS for pain and WOMAC index. The follow up period was 1 year. The authors concluded that RF was significantly better than IA corticosteroids in relieving intra articular pain and that RF injection caused a decrease in synovial fluid concentrations of TNF- α, IL-1 and MMP-3.
Gupta et al.[51] performed a systematic review of published studies investigating conventional, pulsed and cooled radiofrequency ablation in the setting of chronic knee pain. The studies reviewed primarily targeted either the genicular nerves or used an intraarticular approach. They concluded that IA PRF appeared to be a safe and effective method giving promising results up to one year of follow up with minimal complications.
Several studies tried to investigate the effect of PRF on other joints like Liliang et al.[52] Who tested the effectiveness of PRF on hip joint. They agreed with the results of this study in the effectiveness and the safety of the procedure.
Schianchi et al.[17] performed a retrospective study on the effect of PRF in multiple joints including small and big joints. The study included fifty-seven consecutive patients with articular pain of the shoulder, knee, trapezio-metacarpal, and first metatarso-phalangeal joints. Patients with intractable joint pain for more than 6 months were treated with IA PRF using fluoroscopic guidance. Follow up was done at 1, 2, and 5 months. Patients showed significant reductions in pain scores at all three follow-up visits. Success rates were higher in small joints (90% and 82%, respectively) than large ones (64% and 60%, respectively). There were no significant adverse effects or complications. The study recommended additional research including control groups and concluded that PRF is a safe and effective method in treating arthrogenic pain agreeing with our results.
6. Conclusion And Recommendations
IA PRF treatment appears to be safe and beneficial in osteoarthritis related knee pain. Further studies with a larger population and randomized controlled study design are warranted to confirm the positive findings of this study report. It is also recommended to follow up patients' consumption of analgesics pre and post-intervention through consumption diaries.
Acknowledgements
Dr Raghda Hasan Abu Bakr and Dr Asmaa Abd El-Hameed Ahmed, Assistant lectureres in the Biomedical Informatics and Medical Statistics Department, Medical Research Institute, Alexandria University for calculating the sample size and the results of our study.
References
- Zhang Y. and Jordan, J. M., Epidemiology of Osteoarthritis, Clin. Geriatr. Med. 26(3), 355-369 (2010).
- Bliddal H. and Christensen, R., The treatment and prevention of knee osteoarthritis: a tool for clinical decision-making, Expert Opin. Pharmacother. 10(11), 1793-1804 (2009).
- Alshami A. M., Knee Osteoarthritis Related Pain: A Narrative Review of Diagnosis and Treatment, Int. J. Health Sci. 8(1),85-104 (2014).
- Schaible H. G., von Banchet, G. S., Boettger, M., Bräuer, R., Gajda, M., Richter, F., et al., The role of proinflammatory cytokines in the generation and maintenance of joint pain, Ann. N.Y. Acad. Sci. 1193(1), 60-69 (2010).
- Altman R., Asch, E., Bloch, D., Bole, G., Borenstein, D., Brandt, K., et al., Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee, Arthritis. Rheum. 29(8), 1039-1049 (1986).
- Masala S., Fiori, R., Bartolucci, D., Mammucari, M., Angelopoulos, G., Massari, F., et al., Diagnostic and Therapeutic Joint Injections, Semin. Intervent. Radiol. 27(02), 160-171 (2010).
- Figueroa D., Calvo, R., Villalón, I. E., Meleán, P., Novoa, F. and Vaisman, A., Clinical outcomes after arthroscopic treatment of knee osteoarthritis, The Knee. 20(6), 591-594 (2013).
- Carr A. J., Robertsson, O., Graves, S., Price, A. J., Arden, N. K., Judge, A., et al., Knee replacement, The Lancet. 379(9823), 1331-1340 (2012).
- Vallejo R., Benyamin, R. M. and Aliaga, L., Radiofrequency vs. pulse radiofrequency: The end of the controversy, Tech. Reg. Anesth. Pain Manag. 14(3), 128-132 (2010).
- Schiltenwolf M. and Fischer, C., Choi WJ et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain 2011; 152: 481-7, Pain. 152(8), 1933-1934 (2011).
- Erdine S., Bilir, A., Cosman, E. R. and Cosman Jr, E. R., Ultrastructural Changes in Axons Following Exposure to Pulsed Radiofrequency Fields, Pain. Pract. 9(6), 407-417 (2009).
- Chao S.-C., Lee, H.-T., Kao, T.-H., Yang, M.-Y., Tsuei, Y.-S., Shen, C.-C., et al., Percutaneous pulsed radiofrequency in the treatment of cervical and lumbar radicular pain, Surg. Neurol. 70(1),59-65 (2008).
- Byrd D. and Mackey, S., Pulsed radiofrequency for chronic pain, Curr. Pain Headache Rep. 12(1), 37-41 (2008).
- Racz G. B. and Ruiz-Lopez, R., Radiofrequency Procedures, Pain. Pract. 6(1), 46-50 (2006).
- Bogduk N., Pulsed Radiofrequency, Pain Med. 7(5), 396-407 (2006).
- E. Sluijter M. and Imani, F., Evolution and Mode of Action of Pulsed Radiofrequency, Anesth. Pain Med. 2(4), 139-141 (2013).
- M. S. P., Sluijter, M. E. and Balogh, S. E., The treatment of joint pain with intra-articular pulsed radiofrequency, Anesthesiol. Pain Med. 3(2), 250 (2013).
- Chua N. H. L., Vissers, K. C. and Sluijter, M. E., Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review, Acta Neurochir. (Wien.). 153(4), 763-771 (2010).
- Louis L. J., Musculoskeletal Ultrasound Intervention: Principles and Advances, Radiol. Clin. North Am. 46(3), 515-533 (2008).
- Iagnocco A. and Naredo, E., Ultrasound-guided corticosteroid injection in rheumatology: accuracy or efficacy?, Rheumatology (Oxford). 49(8), 1427-1428 (2010).
- Kellgren J. H. and Lawrence, J. S., Radiological Assessment of Osteo-Arthrosis, Ann. Rheum. Dis. 16(4),494-502 (1957).
- McConnell S., Kolopack, P. and Davis, A. M., The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties, Arthritis Rheum. 45(5), 453-461 (2001).
- Bellamy N., WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire, J. Rheumatol. 29(12), 2473-2476 (2002).
- Krebs E. E., Carey, T. S. and Weinberger, M., Accuracy of the Pain Numeric Rating Scale as a Screening Test in Primary Care, J. Gen. Intern. Med. 22(10), 1453-1458 (2007).
- Choi W.-J., Hwang, S.-J., Song, J.-G., Leem, J.-G., Kang, Y.-U., Park, P.-H., et al., Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial, PAIN®. 152(3), 481-487 (2011).
- Chen B., Lai, L., Putcha, N., Stitik, T., Foye, P. and DeLisa, J., Optimal needle placement for ultrasound-guided knee joint injections or aspirations, J Trauma Treat. 3(216), 2167-1222.1000216 (2014).
- Masala S., Fiori, R., Raguso, M., Morini, M., Calabria, E. and Simonetti, G., Pulse-dose radiofrequency for knee osteoartrithis, Cardiovasc. Intervent. Radiol. 37(2), 482-487 (2014).
- Karaman H., Tüfek, A., Kavak, G. Ö., Yildirim, Z. B., Uysal, E., Celik, F., et al., Intra-articularly applied pulsed radiofrequency can reduce chronic knee pain in patients with osteoarthritis, J. Chin. Med. Assoc. 74(8), 336-340 (2011).
- Cohen S. P., Doshi, T. L., Constantinescu, O. C., Zhao, Z., Kurihara, C., Larkin, T. M., et al., Effectiveness of Lumbar Facet Joint Blocks and Predictive Value before Radiofrequency Denervation The Facet Treatment Study (FACTS), a Randomized, Controlled Clinical Trial, Anesthesiology. (2018).
- Jenkinson C., Coulter, A., Bruster, S., Richards, N. and Chandola, T., Patients’ experiences and satisfaction with health care: results of a questionnaire study of specific aspects of care, Qual. Saf. Health Care. 11(4), 335-339 (2002).
- Hendriks A., Vrielink, M., Smets, E., Van Es, S. and De Haes, J., Improving the assessment of (in) patients' satisfaction with hospital care, Med. Care.270-283 (2001).
- Chen A. L., Desai, P., Adler, E. M. and Di Cesare, P. E., Granulomatous Inflammation After Hylan G-F 20 Viscosupplementation of the Knee, J. Bone. Joint. Surg. 84(7),1142-1147 (2002).
- Bernardeau C., Acute arthritis after intra-articular hyaluronate injection: onset of effusions without crystal, Ann. Rheum. Dis. 60(5), 518-520 (2001).
- Roos J., Epaulard, O., Juvin, R., Chen, C., Pavese, P. and Brion, J.-P., Acute pseudoseptic arthritis after intraarticular sodium hyaluronan, Joint Bone Spine. 71(4), 352-354 (2004).
- Charalambous C. P., Tryfonidis, M., Sadiq, S., Hirst, P. and Paul, A., Septic arthritis following intra-articular steroid injection of the knee? a survey of current practice regarding antiseptic technique used during intra-articular steroid injection of the knee, Clin. Rheumatol. 22(6), 386-390 (2003).
- Lequerré T., Nouvellon, M., Kraznowska, K., Bruno, M.-C., Vittecoq, O., Mejjad, O., et al., Septic arthritis due to Actinomyces naeslundii: report of a case, Joint Bone Spine. 69(5), 499-501 (2002).
- Brown H. and Prescott, R. Applied Mixed Models in Medicine. John Wiley & Sons, Ltd; 2006.
- Liu H. and Wu, T., Sample Size Calculation and Power Analysis of Time-Averaged Difference, J. Mod. Appl. Stat. Methods. 4(2), 434-445 (2005).
- Kotz S, Balakrishnan N, Read CB, Vidakovic B. Encyclopedia of statistical sciences. 2nd ed. Hoboken, N.J.: Wiley-Interscience; 2006.
- Kirkpatrick LA, Feeney BC. A simple guide to IBM SPSS statistics for version 20.0. Student ed. Belmont, Calif.: Wadsworth, Cengage Learning; 2013.
- Duymus T. M., Mutlu, S., Dernek, B., Komur, B., Aydogmus, S. and Kesiktas, F. N., Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options, Knee Surg. Sports Traumatol. Arthrosc. 25(2), 485-492 (2017).
- Meheux C. J., McCulloch, P. C., Lintner, D. M., Varner, K. E. and Harris, J. D., Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review, Arthroscopy. 32(3), 495-505 (2016).
- Dieppe P. A. and Lohmander, L. S., Pathogenesis and management of pain in osteoarthritis, The Lancet. 365(9463), 965-973 (2005).
- Straub R. H. and Cutolo, M., Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management, Arthritis Rheum. 56(2), 399-408 (2007).
- Cutolo M. and Straub, R. H., Circadian rhythms in arthritis: Hormonal effects on the immune/inflammatory reaction, Autoimmun. Rev. 7(3), 223-228 (2008).
- Cutolo M., Straub, R. H. and Bijlsma, J. W. J., Neuroendocrine-immune interactions in synovitis, Nat. Clin. Pract. Rheumatol. 3(11), 627-634 (2007).
- Schmidt M., Weidler, C., Naumann, H., Anders, S., Schölmerich, J. and Straub, R. H., Reduced capacity for the reactivation of glucocorticoids in rheumatoid arthritis synovial cells: Possible role of the sympathetic nervous system? Arthritis Rheum. 52(6), 1711-1720 (2005).
- Daniels E. W., Cole, D., Jacobs, B. and Phillips, S. F., Existing Evidence on Ultrasound-Guided Injections in Sports Medicine, Orthop. J. Sports. Med. 6(2), 2325967118756576 (2018).
- Huang Z., Du, S., Qi, Y., Chen, G. and Yan, W., Effectiveness of ultrasound guidance on intraarticular and periarticular joint injections: systematic review and meta-analysis of randomized trials, Am. J. Phys. Med. Rehabil. 94(10), 775-783 (2015).
- Yuan Y., Shen, W., Han, Q., Liang, D., Chen, L., Yin, Q., et al., Clinical observation of pulsed radiofrequency in treatment of knee osteoarthritis, Int. J. Clin. Exp. Med. 9(10), 20050-20055 (2016).
- Gupta A., Huettner, D. P. and Dukewich, M., Comparative Effectiveness Review of Cooled versus Pulsed Radiofrequency Ablation for the Treatment of Knee Osteoarthritis: A Systematic Review, Pain physician. 20(3), 155-171 (2017).
- Liliang P.-C., Liang, C.-L., Lu, K., Chen, Y.-W. and Chye, C.-L., Pulsed radiofrequency treatment of articular branches of femoral and obturator nerves for chronic hip pain, Clin Interv Aging.569 (2015).