Information
Journal Policies
Comparative Study on the Plasmatic CRP Level Variation in Dogs Undergoing Surgery with CO2 Laser and Scalpel Blade Incisions in a Pre- and Post-Surgical Time-Point
L.Miguel Carreira1,2,3*, Levi Silva3, Pedro Azevedo3, Rita Ramalho3, Ricardo Baiao3, Steve Nielsen4
2.Interdisciplinary Centre Research Animal Health (CIISA) – FMV/ULisboa, Av. da Universidade Técnica de Lisboa, Polo Universitário do Alto da Ajuda, 1300-477 Lisbon, Portugal.
3.Anjos of Assis Veterinary Medicine Centre (CMVAA), Rua Dª Francisca da Azambuja Nº9-9A, 2830-077 Barreiro, Portugal.
4.Light Scalpel/ Aesculight/ LuxarCare. 11818 North Creek Parkway North, Ste. 100 Bothell, WA 98011, United States of America.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
C-Reactive Protein (CRP) was the first described acute phase response protein. In the dog, plasmatic CRP level can increase, as soon as, 4 hours after the onset of tissue lesion stimulus, decreasing afterward in a fast way if the underlying cause is resolute. In a peri-operative scenario, plasmatic CRP can also work as good monitoring tool relating to inflammatory tissue level, verifying that the increasing magnitude of its levels is correlated with the surgical trauma intensity produced in the patient. Fifty healthy bitches (N=50) presented for spay elective surgery were randomly distributed in 2 groups, according to the type of surgical incision technique used: scalpel Blade(GB), or CO2 Laser(GL). The study aimed to compare the plasmatic CRP level variation between GB and GL patients. From each patient, two blood samples were collected in 2 different time-points: T0(immediately after catheterization) and T1(4 hours after surgery ends), to measurement the plasmatic CRP level variation. Statistically significant results considered when p< 0.05. Statistically significant differences were registered between the CRP levels variation and the surgical incision techniques (p< 0.01), with CRP levels of GB, tended to increase from T0 to T1 contrary to the GL; and between CRP levels variation and body-weight (p< 0.01). Concluding, it was possible to verify differences between both techniques, with lower plasmatic PCR level variation being registered for patients submitted to CO2 Laser surgery. Thus, the use of CO2 laser system in surgical incisions is associated with lower inflammatory response, promoting a better comfortable peri-operative period for the patient.
Dog; C-Reactive Protein (CRP); Surgery; Inflammatory response; Scalpel Blade incisions; CO2 Laser incisions, Anesthesiology
Whenever signals of a potential hazard to body integrity are recognized, the inflammatory response (IR) is immediately activated and developed, being composed by several cell types and cytokines whose participation aims the healing of damaged tissue [1]. The Acute Phase Response (APR), represents one of several constituent subsystems of the innate immune system, and its presence can be seen in every animal species [2-5]. The APR is responsible for multiple systemic effects, one of them particularly noteworthy, is the modulation of protein synthesis in the liver which results in plasmatic levels changes from a specific group of proteins called Acute Phase Proteins (APPs), whose majority are considered as positive APPs since their plasmatic level increase during the inflammatory process [3-6]. APPs hold as principal roles: 1) microorganisms opsonization and activation of the complement system, 2) scavenging of free radicals and cellular components, and 3) neutralization of the proteolytic enzymes action[4,6]. C - reactive protein (CRP) was the first described APP. It belongs to the Pentraxins family, holds a 100- kDa molecular weight and a pentameric structure with two faces. One face binds to phosphocholine (molecule present in all cell membranes, including bacteria and protozoa), while the other binds to antibody receptors present in the neutrophils’ surface, like FCγRI and FCγRII. Also, CRP can activate the complement system through the classical pathway, acts as an opsonin, and promotes microorganisms and dead or damaged cells phagocytosis [1-3,5]. Despite its pro-inflammatory functions, CRP also has anti-inflammatory abilities, considering that inhibits the neutrophil degranulation and the superoxide production, blocks platelet aggregation and promotes the healing of injured tissues through its capacity to minimize the damage produced during the inflammatory process [1]. In dogs, plasmatic CRP level can increase, as soon as, 4 hours after the onset of tissue lesion stimulus, decreasing afterward in a fast way if the underlying cause is resolute. For this reason, this biomarker can be used as a monitoring tool and prognosis factor for patient clinical condition evaluation [2,3,5-8]. In a peri-operative scenario, CRP can also work as a proper monitoring tool relating to inflammatory tissue level, verifying that the increasing magnitude of its levels is correlated with the surgical trauma intensity produced in the patient[9]. The present study was developed in a sample of 50 healthy bitches (N=50) undergoing to an elective ovariohysterectomy (OVH) surgery and aimed to compare the CRP plasmatic level variation between patients submitted to surgical incisions with CO2 Laser and scalpel Blade considering a pre- and post-surgical time-points.
2. Materials And Methods
The study used a sample of 50 healthy bitches (N=50) presented at Anjos of Assis Veterinary Medicine Centre - CMVAA for elective OVH surgery, and at no time were used as experimental animals. Inclusion criteria were the following: 1) healthy clinical condition, 2) pre-operative blood testing (hemogram, ionogram, liver and kidney function) with values within the reference range, 3) no undergoing treatment, 4) a body condition score of 4 or 5 according to Laflamme (1997)[10] body condition scale, and 5) a signed consent form obtained from the owners to the animal’s participation in the study. The sample was randomly distributed between 2 groups with 25 individuals each (n=25), according to the type of surgical incision used technique: with scalpel Blade (GB) or with CO2 Laser (GL). The same surgeon performed the procedure. In GB surgical incisions were performed with scalpel blade Nº 24, and in GL, surgical incisions were made with the Surgical CO2 Laser System - Aesculight 1507 Surgical Laser System - Aesculight ®. A Superpulse energy emission mode, a laser focus of 0,4 mm2 and a power output of either 12 Watts (W) or 15 W were selected. All patients were catheterized via the cephalic vein after topical application of bupivacaine gel 5% and received a continuous intravenous fluid therapy with saline (2 ml/kg/h NaCl 0.9%). A presurgical protocol with amoxicillin + clavulanic acid (10 mg/kg IM), carprofen (4 mg/kg); buprenorphine (0,005 mg/kg); acepromazine (0,005 mg/kg) was used for all patients. For anesthetic induction, it was used propofol (4 mg/kg), and isoflurane for the maintenance of anesthesia. Two peripheral blood samples of 1 ml volume each, were collected in every patient on two different time-points: T0 (immediately after catheterization) and T1 (4 hours after the end of surgery), using the previously catheterized vein. The 1st blood sample (T0) was divided into an EDTA tube to perform hemogram, and into a Heparin tube which was then centrifuged for 3 minutes at 9000 rotations per minute (r.p.m.), using the resulting plasma for the CRP level measurement. The 2nd blood sample (T1), was collected 4 hours after the end of surgery during the catheter removal and centrifuged in an equal manner as described for T0 to measure the plasmatic CRP level. To quantify the plasmatic CRP level was used a canine-specific dry chemistry assay for in-house CRP measurement (FUJI DRI CHEM SLIDE vc-CRP-P, FUJIFILM®). During surgery, data collection of the following parameters: cardiac frequency (BPM), respiratory frequency (CCP), Systolic Arterial Pressure (SAP), Diastolic Arterial Pressure (DAP), Mean Arterial Pressure (MAP), EtCO2, sPO2, and oesophageal temperature, was made using a multiparametric anesthetic monitor. Statistical analysis was performed with Rstudio and RCommander computer software. Through the Shapiro-Wilk test, we verified that the majority of the studied variables did not possess a normal distribution. Therefore, for the study of correlation coefficients between the different parameters we used the Spearman test; for the analysis of differences in the variation of CRP concentrations depending on the used type of incision technique (as well as other parameters), we used Wilcoxon test, Student t-test, and a Mixed Linear Model followed by a Type II Wald Chi-Square test. The obtained results were considered statistically significant with a 95% confidence interval and when p < 0.05.
3. Results
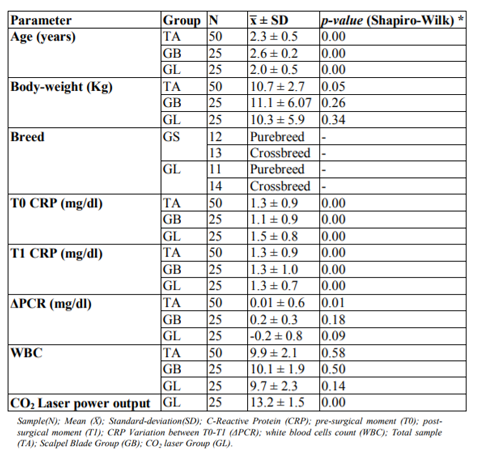
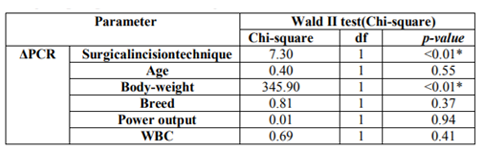
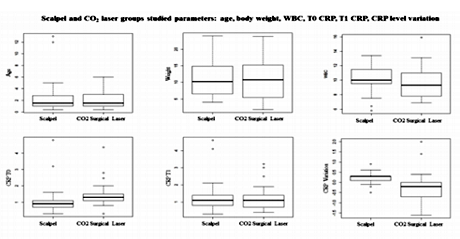
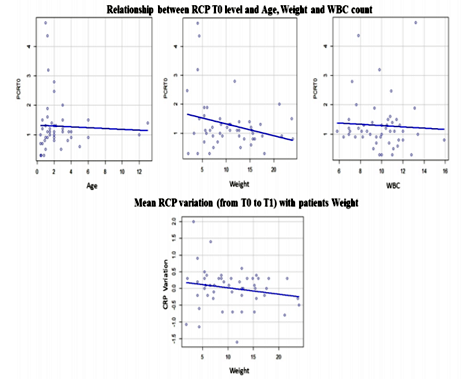
Descriptive statistics of age, body weight, breed, CRP level at T0 and T1, CRP level variation (Δ), White Blood Cells (WBC) and CO2 Laser system power used, from total sample (TS), GB and GL groups are listed at Table 1 (Figures 1, 2, 3). The relationship between the CRP level and: surgical incision technique, age, body weight, breed, CO2 Laser system power used and WBC count was analyzed (Table 2).
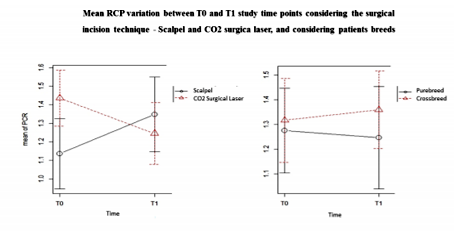
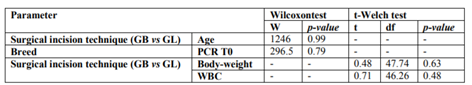
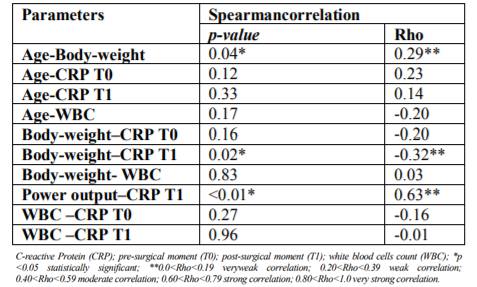
Statistically significant differences were registered between the CRP levels and the surgical incision techniques (p < 0.01). It was possible to verify that at T0, GL had higher CRP levels mean than GB; however, at T1 (4 hours after surgery) it was observed that CRP levels of GB tended to increase contrary to the GL (Figure 7). Considering the surgical incision technique, no statistically significant differences were registered between both groups regarding age (p = 0.99), body weight (p = 0.63) and WBC count (p = 0.48) (Table 3). The relationship between CRP levels and body weight were statistically significantly different (p < 0.01), with heavier patients presenting lower CRP levels at T1 (Figure 8). No, statistically significant differences were obtained in relationships between the CRP levels and age (p=0.55), breed (p=0.37), CO2 Laser power system (p=0.94) and WBC count (p=0.41). Although no statistically significant difference (p=0.37) was registered between CRP level and dog breeds, it was possible to verify that CRP levels tended to increase after surgery in purebred patients and to decrease in crossbreed (Figure 9). Correlation coefficients analyses were performed between several parameters (Table 4). Although weak, a positive correlation was found between the patients’ age and body-weight (p=0.04; Rho=0.29), and between body-weight and the CRP levels at T1 but in a negative way (p=0.02; Rho=-0.32). A strong and positive correlation was identified between the parameters CO2 Laser power system and T1 CRP levels (p=0.001; Rho=0.63).
4. Discussion
Canine CRP is a positive APP whose plasmatic levels can change with multiple factors in physiological and pathological conditions. Several authors have established different CRP level mean values in physiological conditions ranging from 0, 02 mg/dl to 1,60 mg/dl [2,9,11-13]. In pathological conditions, CRP increases can be observed as soon as 4 hours after the tissue injury stimulus, reaching up until 100 times higher than its normal value in a 24-48 hours period after the injury [2,5,7,8]. In the study, patients from TS, GB, and GL presented a T0 plasmatic CRP level mean (before surgical stimulus) in accordance with the published studies, and very similar between them (TS = 1.3 ± 0.9; GB = 1.1 ± 0.9; GL = 1.5 ± 0.8).
Just like other APPs, the CRP synthesis starts when the body is exposed to different injury stimulus that induces an inflammatory response, such as surgical trauma. Several studies performed in dogs suggest that the CRP level should be included as a post-surgical systemic inflammation biomarker, considering that it levels directly correlate with the surgical trauma level produced on the patient [9,14-16]. Thus, the more minimally invasive the surgical procedure will be, the minor trauma will induce in the body resulting therefore in a lower inflammatory response. It has been reported that the CO2 Laser surgery is related to a lesser inflammatory response since it produces less hemorrhage, edema, and pain due to its ability to vaporize the tissues hit by its energy [17-24]. Aside from that, it can seal small blood vessels, lymphatics, and nerve endings, resulting in better control of the pro-inflammatory stimulus caused by the surgical procedure [24-28]. Therefore, it would expect that the CO2 Laser surgery would produce lower increases in plasmatic CRP level after the surgical procedure when compared to surgery using the scalpel blade. The results of our study are in accordance with this, considering that it was possible to verify a statistically significant difference between the plasmatic CRP level and the type of surgical incision technique used – scalpel blade and CO2 Laser (p < 0.01). Also, it was observed that the GL presented a lower plasmatic CRP mean level comparing with the GB; and that the plasmatic CRP level mean between T0 and T1 tended to decrease in GL and to increase in GB. No statistically significant differences were register for the studied parameters: age (p=0.99), body weight (p = 0.63) and WBC count (p = 0.48) between the GB and GL. Similar for the parameter breed, considering that the GB had 12 purebreds and 13 crossbreed individuals, and the GL had 11 purebred and 14 crossbreed animals.
Plasmatic CRP levels were not affecting by the carprofen pre-surgically administered to each patient, considering that the non-steroidal anti-inflammatory drugs act ahead of the cyclooxygenase (COX) system. The CRP synthesis is induced by pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which in the context of the inflammatory cascade are produced before the COX system. Considering this is possible to conclude that the plasmatic CRP level registered in the study was not affected by the studied parameters (age, body weight, breed, WBC, and pre-surgical protocol), but only due to the type of surgical incision technique used in the patients.
No correlation between age and the plasmatic CRP level in GB and GL were registered considering that T0 CRP level presented a p=0.12 and Rho=0.23. The results are in accordance with the published studies that conclude that there is no correlation between age and the plasmatic CRP level in healthy dogs [11,29]. No statistically significant differences were identified in the relationship between age and the WBC count (p=0.17 and Rho=0.2). Despite that, it was possible to verify that the WBC counts tended to be lower as the patients were older, which is following the study Brenten et al. (2016) that concluded the WBC counts presented a negative correlation with the patients’ age [30].
The impact of purebred on the incidence of specific diseases is well known, being described until this moment more than 370 hereditary diseases, associated with some breeds [31]. The Miniature Schnauzer is an example of that, whose CRP normal level is higher than individuals from other breeds, possibly due to a higher predisposition for atherosclerosis development[32]. However, in our study, it was not possible to register statistically significant differences in the plasmatic CRP levels between the different breeds included in our sample. The insufficient number of purebred individuals used in the sample could explain this difference in the results. To our knowledge, until this moment there is no study comparing the normal CRP levels between purebred and crossbreed dogs. According to the obtained results in our study, no statistically significant differences were registered between the plasmatic CRP level and the pure-and crossbreed individuals (p=0.79). Despite that fact, it was possible to verify an increase in the plasmatic CRP level in purebred individuals, and a decrease in crossbreed individuals, which however was not statistically significant (p=0.37). Perhaps in future, a study with a more significant sample would allow us to establish a significant difference between pure- and crossbreed groups.
Regarding the type of surgical incision technique used, the sample was randomly distributed between 2 groups: with a scalpel blade (GB) and with CO2 Laser (GL). The CO2 surgical laser uses the energy produced by the CO2 laser beam, which is highly absorbed by water (the main component of tissues), promoting the cellular vaporization through the generated photothermic effect [33]. The CO2 laser energy can be used through 3 types of energy emission modes: continuous, pulsed and superpulse. The superpulse mode reduces the exposure time of tissues to the CO2 Laser energy by just a few milliseconds, while providing high energy levels through each emission. It has the advantage of allowing the cooling of tissue hit by the laser beam between each energy emission, and consequently decrease the level of thermal injury produced to the adjacent tissue [23,24,34]. Selecting the power output of the surgical laser system is a factor that influences the photothermic interaction between the CO2 laser and the target tissues. In 1995, Wilder-Smith et al. [35], verified a rise in temperature on tissues surrounding the incision line made by the CO2 laser as the power output of the system increased. This could be explaining by the fact that high power output results in higher locally generated heat, surpassing at some point the heat dissipation rate that occurs through the adjacent tissue. Considering this is expecting the development of higher inflammatory response in the tissue associated with possible thermal injury areas caused by higher power output values selected on the CO2 laser system. However, in our study no statistically significant differences were registered between the plasmatic CRP levels and the power output selected (p=0.94). These results could be related with the fact that only two different power output values were selected – 12 and 15 W. It is possible that this range of values was too short to allow us to verify significant variations regarding the thermal injury in the adjacent tissues to the incision line hit by the laser beam. Therefore, if a broader range of power output values was used, we could have obtained different results. Moreover, by always using the superpulse energy emission mode it might have influenced the obtained results since in this mode the CO2 Laser energy hits the tissues is briefly interrupted, allowing dissipating the locally generated energy along the adjacent tissues. By considering this, it is possible that if using a continuous energy emission mode, different results could be verifying when relating the plasmatic CRP levels and the selected power output. Regarding the relationship between WBC count and plasmatic T0 CRP level, no statistically significant results were obtained (p=0.27 and Rho=-0.16). The results are not in accordance with those from Nakamura et al. (2008) [8], which identified a weak positive correlation between WBC counts and plasmatic CRP levels, verifying that the WBC counts above the reference range were correlated with increases in plasmatic CRP levels. This differences between results might be associated with the facts that no animals in our sample had WBC counts above the reference range, and the sample was composed only by females, contrary to the sample used by Nakamura et al. (2008)[8] that used animals of both genders, being known that males have higher WBC counts relatively to females [35].
5. Conclusion
Being the plasmatic CRP an important biomarker of the inflammatory response, by measuring it variation in two peri-operative periods (T0- before surgery, and T1- 4 hours after the end of surgery) in patients submitted to surgical incisions made with CO2 Laser system and with scalpel blade, it was possible to verify statistically significant differences between both techniques, with lower plasmatic CRP levels being registered for patients submitted to CO2 Laser surgery. Thus, the use of CO2 laser system in surgical incisions is associated with the lower inflammatory response of the incised tissues, promoting a better comfortable peri-operative period for the patient.
Acknowledgments
The authors thank to Centre for Interdisciplinary Research in Animal Health (CIISA) – Faculty of Veterinary Medicine, University of Lisbon (FMV/ULisboa), Lisbon - Portugal, and Anjos of Assis Veterinary Medicine Centre (CMVAA), Barreiro – Portugal.
References
- Tizard, I. (2012) Veterinary immunology. (9th edition). St. Louis, Missouri: Elsevier.
- Eckersall, P. D., & Conner, J. G. (1988). Bovine and canine acute phase proteins. Veterinary Research Communications, 12(2–3), 169–178.
- Cerón, J. J., Eckersall, P. D., & Martínez-Subiela, S. (2005). Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Veterinary Clinical Pathology, 34(2), 85–99.
- Gruys, E., Toussaint, M. J. M., Niewold, T. A., & Koopmans, S. J. (2005). Acute phase reaction and acute phase proteins. Journal of Zhejiang University SCIENCE, 6B (11), 1045– 1056.
- Cray, C., Zaias, J., & Altman, N. H. (2009). Acute phase response in animals: a review. Comp Med, 59(6), 517–526.
- Paltrinieri, S. (2007). Early biomarkers of inflammation in dogs and cats: The acute phase proteins. Veterinary Research Communications, 31(SUPPL. 1), 125–129.
- Caspi, D., Baltz, M., Snel, F., Gruys, E., Niv, D., Batt, R. M., Munn, E. A., Buttress, N., Pepys, M. B. (1984). Isolation and characterization of C-reactive protein from the dog. Immunology, 53(2), 307–313.
- Nakamura, M., Takahashi, M., Ohno, K., Koshino, A., Nakashima, K., Setoguchi, A., Fujino, Y., Tsujimoto, H. (2008). C-reactive protein concentration in dogs with various diseases. The Journal of Veterinary Medical Science / the Japanese Society of Veterinary Science, 70(2), 127–131.
- L.Miguel Carreira & Simão Boal. (2015). Serum and synovial fluid C-reactive protein level variations in dogs with degenerative joint disease and their relationships with physiological parameters. Veterinary Research Communications,39(3):163-169. DOI: 10.1007/s11259-015-9640-7
- Yamamoto, S., Shida, T., Miyaji, S., Santsuka, H., Fujise, H., Mukawa, K., Nagae, T., Naiki, M. (1993). Changes in serum C-reactive protein levels in dogs with various disorders and surgical traumas. Veterinary Research Communications, 17(2), 85–93.
- Laflamme, D. P. Development and validation of a body condition score system for dogs. Canine Practice, 22, 10-15
- Yamamoto, S., Shida, T., Okimura, T., Otabe, K., Honda, M., Ashida, Y., Sarikaputi, M., Naiki, M. (1994). Determination of C-reactive protein in serum and plasma from healthy dogs and dogs with pneumonia by ELISA and slide reversed passive latex agglutination test. The Veterinary Quarterly, 16(2), 74–77.
- Yamashita, K., Funjianga, T., Miyamoto, T., Hagio, M., Izumisawa, Y., Kotani, T. (1994). Canine acute phase response: relationship between serum cytokine activity and acute phase proteins in dogs. Journal of Veterinary Medical Science, 56(3), 487–492.
- Otabe, K., Sugimoto, T., Jinbo, T., Honda, M., Kitao, S., Hayashi, S., Shimizu, M., Yamamoto, S. (1998). Physiological levels of C-reactive protein in normal canine sera. Veterinary Research Communications, 22(2), 77–85.
- Sibanda, S., Hughes, J. M. L., Pawson, P. E., Kelly, G., & Bellenger, C. R. (2006). The effects of preoperative extradural bupivacaine and morphine on the stress response in dogs undergoing femoro-tibial joint surgery. Veterinary Anaesthesia and Analgesia, 33(4), 246–257.
- Stedile, R., Beck, C. A. C., Schiochet, F., Ferreira, M. P., Oliveira, S. T., Martens, F. B., Muccillo, M. S. (2009). Laparoscopic versus open splenectomy in dogs. Pesquisa Veterinária Brasileira, 29(8), 653–660.
- Michelsen, J., Heller, J., Wills, F., & Noble, G. (2012). Effect of surgeon experience on postoperative plasma cortisol and C-reactive protein concentrations after ovariohysterectomy in the dog: A randomised trial. Australian Veterinary Journal, 90(12).
- Romanos, G., Siar, C. H., Ng, K., & Toh, C. G. (1999). A preliminary study of healing of superpulsed carbon dioxide laser incisions in the hard palate of monkeys. Lasers in Surgery and Medicine, 24(5), 368–374.
- Clark, G. N., & Sinibaldi, K. R. (1994). Use of a caron dioxide laser for treatment of elongated soft palate in dogs. J Am Vet Med Assoc. 11, 1779-1781.
- Dye, T. L., Teague, H. D., Ostwald, D. A., & Ferreira, S. D. (2002). Evaluation of a technique using the carbon dioxide laser for the treatment of aural hematomas. Journal of the American Animal Hospital Association, 38(4), 385–390.
- Shelley, B. A. (2002). Use of the carbon dioxide laser for perianal and rectal surgery. The Veterinary Clinics of North America. Small Animal Practice, 32(3), 621–37.
- Dunié-Mérigot, A., Bouvy, B., & Poncet, C. (2010). Comparative use of CO2 laser, diode laser and monopolar electrocautery for resection of the soft palate in dogs with brachycephalic airway obstructive syndrome. Veterinary Record, 167(18), 700–704.
- Paczuska, J., Kiełbowicz, Z., Nowak, M., Antończyk, A., Ciaputa, R., & Nicpoń, J. (2014). The carbon dioxide laser: an alternative surgery technique for the treatment of common cutaneous tumors in dogs. Acta Veterinaria Scandinavica, 56(Table 2), 1.
- Carreira, L. M., & Azevedo, P. (2016). Comparison of the influence of CO2-laser and scalpel skin incisions on the surgical wound healing process. ARC Journal of Anesthesiology, 1(3), 1–8.
- Carreira, M. L., Ramalho, R., Nielsen, S., & Azevedo, P. (2017). Comparison of the hemodynamic response in general anesthesia between patients submitted to skin incision with scalpel and CO2 laser using dogs as an animal model. A preliminary study. ARC Journal of Anesthesiology, 2(1), 24–30.
- Fisher, I. E., Frame, J. W., Browne, R. M., & Tranter, R. M. D. (1983). A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa. Archs Oral Biol, 28(4), 287–291.
- Holt, T. L., & Mann, F. A. (2002). Soft tissue application of lasers. Veterinary Clinics of North America - Small Animal Practice, 32(3), 569–599.
- Bartels, K. E. (2002). Lasers in veterinary medicine—where have we been, and where are we going? Vet Clin Small Anim, 32, 495–515.
- Palmer, S. E. (2002). Treatment of common cutaneous tumors using the carbon dioxide laser. Clinical Techniques in Equine Practice, 1(1), 43–50.
- Kuribayashi, T., Shimada, T., Matsumoto, M., Kawato, K., Honjyo, T., Fukuyama, M., Yamamoto, Y., Yamamoto, S. (2003). Determination of serum C-reactive protein (CRP) in healthy beagle dogs of various ages and pregnant beagle dogs. Experimental Animals / Japanese Association for Laboratory Animal Science, 52(May), 387–390.
- Brenten, T., Morris, P. J., Salt, C., Raila, J., Kohn, B., Schweigert, F. J., & Zentek, J. (2016). Age-associated and breed-associated variations in haematological and biochemical variables in young labrador retriever and miniature schnauzer dogs. Veterinary Record Open, 3(1).
- Fleischer, S., Sharkey, M., Mealey, K., Ostrander, E. A., & Martinez, M. (2008). Pharmacogenetic and metabolic differences between dog breeds: their impact on canine medicine and the use of the dog as a preclinical animal model. The AAPS Journal, 10(1), 110– 119.
- Wong, V. M., Kidney, B. A., Snead, E. C. R., Myers, S. L., & Jackson, M. L. (2011). Serum C-reactive protein concentrations in healthy Miniature Schnauzer dogs. Veterinary Clinical Pathology, 40(3), 380–383.
- Sawish, T. J. (2011). Oral Surgery for general practitioner. Principles and Practice of Laser Dentistry
- Patel, A. (2013). Laser Airway Fire Protocol. Benumof and Hagberg's Airway Management.
- Amit Kherac; Darren K.McGuire, Sabina A.Murphy, Harold G.Stanek, Sandeep R.Das, Wanpen Vongpatanasin, Frank H.WiansJr, Scott M.Grundy, James A.de Lemos. 2005. Race and Gender Differences in C-Reactive Protein Levels. Journal of the American College of Cardiology. 46 (3):464-469.