Information
Journal Policies
Cardiopulmonary Bypass: Basic Principles and Updates in Anaesthetic Management
Singha SK1, Karim HMR, Panda CK3
2.MD, DNB, IDCCM, Assistant Professor, Department of Anaesthesiology and Critical Care, All India Institute of Medical Sciences, Raipur, India
3.MD, PDF (Crit Care), Assistant Professor, Department of Anaesthesiology and Critical Care, All India Institute of Medical Sciences, Raipur, India
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Open heart surgeries are becoming widely available now a day. However, specialized anaesthesiologists are not that widely available yet. Moreover, the post graduate curriculum in anaesthesiology also needs basic training and education in such cases. Cardiopulmonary bypass management is very important. Therefore the basic knowledge of the principles of cardiopulmonary management is also essential. The present write-up (review) is intended for the postgraduate trainees in anaesthesia and non-cardiac anaesthesiologist practitioners who need to mange such cases due to unavoidable circumstances.
Anaesthesia, Education and Training, Cardiopulmonary Bypass, Postgraduate education, Anesthesiology
Cardiopulmonary bypass (CPB) is a medically supervised technique or procedure in which blood is temporarily diverted through a heart lung machine to maintain the circulation, oxygenation and ventilation (i.e. carbon-di-oxide elimination) for the body. By incorporating an extracorporeal circulation and supporting the functionality of heart and lung, CPB techniques allows open heart surgeries to be performed in bloodless and even motionless heart. The use of CPB is however not limited to the open heart surgeries or cardiac surgeon and anaesthesiologists only[1]. Although CPB machine and circulation is managed by a perfusionist, anaesthesiologists are very well related to the CPB and bypass circulation. They need to be not only vigilant but also have to actively modify the anaesthetic and intraoperative management of the patient. CPB associated pathophysiologic changes like hypothermia, haemodilution, inflammatory responses alter the pharmacokinetics of the anaesthetic drugs[2]. Monitoring the adequacy of anaesthesia during bypass circulation is an up-heal task. Therefore a basic knowledge of the CPB technique, working principle and possible interactions of anaesthetic management is necessary for a cardiac anaesthesiologist. Although cardiac anaesthesia has emerged as a separate branch, limited availability of such specialist especially in developing countries still force non cardiac anaesthesiologist to face the situation even for cardiac surgeries with limited prior exposure to managing patients on cardiopulmonary bypass. The present lesson is prepared keeping these in mind.
2. Review Objectives
The present write up is prepared with the objectives of briefly discussing the
1. Basic structure and principle of working of CPB machine and circuit
2. CPB circuitry and circulation of blood
3. Principles of CPB practice
4. Anaesthetic implication and management
5. Take home message (Conclusion)
The development of cardiopulmonary bypass machine and technique can be said as development of John Gibbon’s Heart Lung machine Model I built by International Business Machines (IBM) laboratories in 1949. Although the first concept of an artificial circulation was formulated by Le Gallois in 1812,[3] the first successful clinical use of CPB (artificial circulation) for cardiac surgery is attributed to by John Gibbon Jr. The results of initial days CPB use were disheartening. The practical clinical use of CPB really began 1955 when two groups led by John Kirklin (using modified Model II) at the Mayo Clinic and C Walton Lillehei at the University of Minnesota, initiated the routine use of it for open heart surgeries[4]. The advancement of extracorporeal circulation techniques has played an essential role and steady progress has been made in cardiopulmonary bypass since it was first conceived[5].
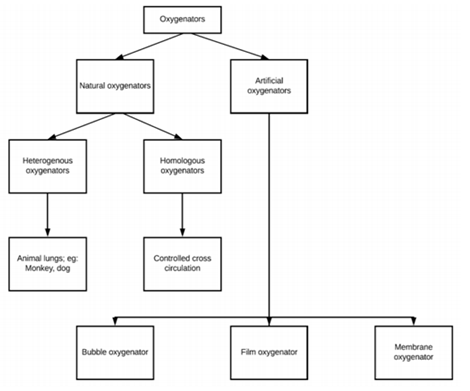
In basics, the cardiopulmonary bypass machine and circuitry are blood reservoirs, blood pumps, and gas exchangers, safety measures along with different ports for gas and drug delivery and exhaust etc. One of the important components of the CPB circuit is the gas exchanger (oxygenators). The gas exchanger has evolved from disc/film oxygenator to bubble oxygenator in to the presently used membrane oxygenator. Gas-exchanger works like an artificial lung giving a contact surface like alveoli for exchange of oxygen and carbon-di-oxide. Membrane oxygenators are sheets and hollow fibre oxygenators made up of polypropylene or silicon. Silicon membrane is regarded as a true membrane[6]. Gas exchange obeys the Fick’s law of diffusion which states that, ‘the amount of gas diffused across a membrane is proportional to the difference of the partial pressure across the membrane, membrane area of contact, and the diffusion constant of the gas whereas it is inversely proportional to the membrane thickness.’ As the surface area of membrane oxygenator is very less than our lung, the difference of the partial pressure across the membrane is made higher by supplementing higher fraction of oxygen to cope up with. The development of oxygenators is shown in Figure 1. (Appended below)
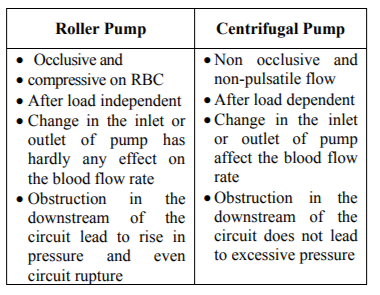
Blood pumps of the cardiopulmonary bypass machine are another important component. The blood pumps works on the basis of Hagen Poiseuille law of flow rate. Currently two types of pumps are used. They are having their own merits and demerits. Centrifugal pumps are regarded as better[7]. Few features are presented in the Table no 1 below:
Safety mechanisms built into the CPB circuit such as bubble detectors, pressure monitors, and oxygen fail safe monitors made the conduct of CPB safe for the patients over a period of time. Besides that there are concerns such as haemodilution, institution of hypothermia and use of heparin without the same the conduct of CPB is almost impossible to imagine. Cardiopulmonary circuit accomplish the following functions:[8]
a. Divert blood from the patient in order to provide optimal surgical conditions,
b. Oxygenate and remove carbon dioxide from the blood,
c. Cool and rewarm the patient's blood and body where appropriate, and
d. Return blood to the patient.
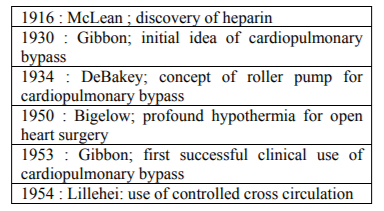
The key early events in the development of extracorporeal circulation are given below in the Table no: 2
Before setting up the circuitry, the keys points needs to be ticked before initiation of CPB. The steps are:
1) Anticoagulation with heparin: Allow for at least 3 -5 minutes customarily for systemic circulation and onset of effect.
2) Vascular cannulation: Arterial and venous cannulation.
3) Arterial cannulation is generally established before venous cannulation. The preferred site for arterial cannulation is the ascending aorta. This is because of multiple reasons like; a) it is easily accessible, b) it doesn’t require an additional incision, c) Ascending aorta can accommodate a larger cannula to provide greater flow at a reduced pressure, and d) it carries a lower risk of arterial wall dissection as compared with other cannulation sites (i.e. femoral or iliac arteries).
4) Venous cannulation can be achieved using a single atrial cannula that is inserted into the right atrium and directed inferiorly into the inferior vena cava (IVC). Drainage holes are located in the inferior vena cava and the right atrium to drain blood from lower extremity and the superior vena cava (SVC) & coronary sinus respectively. The other approach being the bicaval cannulation where the access to right atrium is required and it involves cannulating the SVC & IVC .Loops placed around the great veins are tightened to divert blood from the right atrium but the blood returning from the coronary sinus will not be drained and hence needs an additional vent is necessary.
5) Careful monitoring for the pre-bypass or rather perioperative period with the help of transoesophageal echocardiography and other routine monitors in place.
6) Protection of heart (myocardium) is of paramount importance. Hypothermia, and use of cardioplegia for diastolic arrest of the myocardium to be in place before bypass.
7) Last but not the least, supplemental medications and inspection of head and neck particularly in reference to symmetry, venous drainage (neck vein and conjunctiva engorgement) and pupil equality is reasonable to serve as a baseline for the anesthetic state.
Once all preparatory steps have been taken, the perfusionist progressively increases the delivery of oxygenated blood to the patient’s arterial system, as systemic venous blood is diverted from the patient’s right side of the heart. Aorta is punctured with a trocar based cannula and cardioplegia cannula is placed.
Cardiopulmonary bypass circulation is started once the systemic perfusion pressures are satisfactory, aortic cross clamp done and cardioplegia is injected. Field suction also collects blood. These bloods from the suction and ventricular drain are pumped by two different pumps to a single cardiotomy reservoir. From this cardiotomy reservoir blood is drained to venous reservoir.
Circulation: A systematic blood pump pumps the blood from the venous reservoir through the heat exchanger followed by gas exchanger. The oxygenated blood coming out gas exchanger is diverted in to two channels. A small channel takes blood through the cardioplegia pump and mixes it with cardioplegia solution.
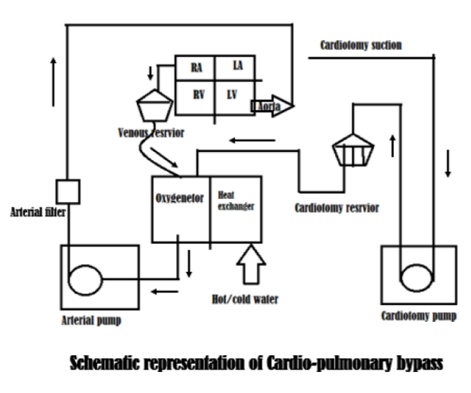
This blood cardioplegia line is then connected to the cardioplegia cannula placed in the aortic sinus (below the aortic cross clamp). The major portion of the oxygenated blood coming out of gas exchanger is pumped in to the aortic cannula through an arterial filter attached in the line and delivers the oxygenated blood above the aortic cross clamp in to the systemic circulation. When this blood returns in to the venous circulation, the cannula with openings in to the SVC and IVC drains the blood in to the venous reservoir again (by gravitational force) till the bypass process is active. A schematic presentation of CPB circuitry is presented in figure 2 below;
The essential components of the clinical extracorporeal circuit are a pump (artificial heart), an oxygenator (artificial lung), a reservoir and the tubings to connect these devices, although systems are now developing without the traditional reservoir. Additional components include a heat exchanger, a system for myocardial protection, and gas and emboli filters. Secondary suction circuits may be added for salvaging shed blood and venting the heart. Centrifugal pumps in use in day to day practice are durable, compact and cause minimal hemolysis compared to the bubble oxygenators. Extensive clinical experience as well as substantial published evidence supports the value of heparin-based bio surfaces for thromboresistane and biocompatibility of the extracorporeal circulation. During the conduct of CPB, it is to be ascertained that the organs of the body are supported by the extracorporeal circuit are adequately perfused with oxygenated blood by continual monitoring of blood flow rate, perfusion pressure, acid-base state, oxygen consumption, coagulation and renal function. Protocol to initiate Cardiopulmonary Bypass: HADDSUE (Heparin, ACT in target, Drugs supplementation, Drips, Swan – pulmonary artery catheter, Urine, Emboli check)[7] (for details see the cited topic / chapter)
In the practice of cardiopulmonary bypass, following aspects needs attention
a. Perfusion pressure: During hypothermia, the lower limit of cerebral auto regulation may be as low as 20 mmHg[9]. However, a mean arterial pressure of 50 to 70 mmHg is usually targeted. This pressure requirement may be higher for elderly, atherosclerotic or chronic hypertensive patients.
b. Pump flow: The objective behind the flow
determination is to maintain the microcirculation. Most of the microcirculation gets perfused when pump flows of 1.2 L/min/m2 is used in patients with haemoglobin of 7 gm% (heamatocrit 22%) and hypothermia of CPB. However, renal perfusion is maintained at higher flow of 1.6 L/min/m2. However, during the period of higher oxygen consumption and at lower hematocrits these flows may become inadequate to meet the oxygen delivery and perfusion and higher flow rates of around 2.2 L/min/m2 would suffice in most of the scenarios.
c. Pulsatility: Although normal physiological perfusion of the human body is pulsatile, current evidence shows conflicting data on the superiority of pulsatile flow as compared to non-pulsatile flow[10].
d. Temperature monitoring: As hyperthermia can increase the ischemic injury to the brain and hypothermia can protect, it is very important to monitor the temperature during CPB. There is substantial variability and lack of concordance in central temperature readings measures in every patient, and investigators recommended the use of at least three measures of core temperature. Core temperature can be monitored from the tympanic membrane, esophagus, nasopharynx, pulmonary artery etc[11].
e. Gas and electrolyte management: Acid base management: both pH- stat and α-stat approach is used for acid base management. The pH-stat approach to acid-base balance maintains a pH of 7.40 and PCO2 of 40 mmHg when corrected for patients body temperature[12]. This typically requires CO2 supplementation during hypothermic CPB. Although it was believed that potent vasodilatory effect of CO2 will increase cerebral blood flow (luxury perfusion) and protect the brain from injury, it has failed to prove so. On the other hand, in the α-stat blood gas management, in which pH is uncorrected for temperature kept at 7.40 with the PCO2 at 40 mmHg[12]. Under conditions of alpha-stat management cerebral autoregulation is maintained, allowing the coupling of cerebral blood flow to cerebral metabolic rate of oxygen while this is uncoupled in pH stat approach although it has been shown to increased jugular vein oxygen concentration as well as
cerebral oxygenation by infrared spectroscopy.[13-15] Murkin et al., in a double blind randomised comparison of alpha-stat and pH-stat management during CPB, found that cognitive dysfunction at two months was less prevalent in patients managed with alpha-stat than with pH-stat strategy (27 versus 44 %)[13]. Presently α-stat blood gas management is used / preferred.
Separation from CPB: Checklist for Weaning from Cardiopulmonary Bypass: Warm – 37 degree celcius, Rhythm- sinus with normal rate of 70 – 90/min, Monitors, Ventilator, Perfusion (can be remembered by- Very WaRM Patient)
[7] (for details see the cited topic / chapter)
The requirements of the ideal cardiac anaesthetic are hemodynamic stability, appropriate analgesia and lack of patient awareness although the last mentioned requirement is a bit difficult to quantify and maintain. Knowledge of the relevant pathophysiology and pharmacology is more important than the employment of a standard cardiac anaesthetic. The effects of cardiopulmonary bypass on drug concentrations are variable and complex. Transesophageal echocardiography is becoming an increasingly useful tool in intraopertive monitoring of hemodynamics and cardiac morphology. The evolution of ‘fast track’ management, which requires early post-operative extubation, requires modification of both CPB and anaesthetic techniques.
Priming of CPB circuit leads to haemodilution which on the other hand leads to dilution of anaesthetic gases as well as drugs. Multiple clinical factors influence pharmacokinetics of anaesthetics during CPB. Effects of haemodilution, altered plasma protein binding, volume of distribution, altered drug clearance, acid-base disturbances, lung isolation, drug sequestration, systemic inflammatory response influence the pharmacokinetics of intravenous anaesthetics while altered blood/gas partition coefficient, altered tissue solubility and oxygenator design influence the pharmacokinetics of inhalational anaesthetics[2]. Besides the above mentioned key factors other factors that alter the drug pharmacokinetics are material used to construct the bypass circuits and oxygenator (some drugs such as isoflurane, propofol, fentanyl; bind avidly to CPB circuits thereby reducing their concentrations), mode of drug administration, volume of distribution of the drug and effects CPB on the ability of liver and kidneys to clear the drug post-operatively. On the other hand, CPB associated hypothermia and impaired cerebral function play major roles in the pharmacodynamics of inhalational and intravenous anaesthetics[2]. The anaesthesiologist faces the challenges with regard to all the basic components i.e. amnesia, analgesia and relaxation. Volatile anaesthetic delivery during CPB is challenging.
Although there is a practice of delivering volatile anaesthetic in to CPB circuit, its quantification, delivery and adequacy cannot be well predicted or measured. So, over and above volatile anaesthetics supplementation, addition of benzodiazepines, opoids, propofol etc., are common practice. Although the patients are more prone to awareness and variable depth of anaesthesia, anaesthetic depth monitoring like bispectral index is not yet recommended as standard of care by American Society of Anesthesiologist and should be considered case by case[16]. Ensuring analgesia during bypass is another challenge as there is lack of monitoring system for adequate analgesia. The classical clinical methods based on the haemodynamics parameters are not reliable or being controlled during CPB. Similarly, muscle relaxation monitoring is also problematic as muscle strength decreases substantially during hypothermia, even in the absence of muscle relaxants[17].
Several non-anaesthetic agents are administered intraoperatively by anaesthesiologists and perfusionists including various electrolytes and inotropes. Calcium, magnesium and bicarbonates are physiologicallly ubiquitous, have important functions and each are subject to fluctuations in the setting of cardiac disease and CPB. Inotropic agents, such as beta agonists phosphodiesterases inhibitors, have an important role in the management of low output states and in aiding separation from CPB.
3. Conclusion
Although CPB machine and circuitry works well as temporary heart lung substitute during CPB phase of open heart surgeries, it cannot substitute the need of vigilant anaesthesiologist yet. CPB is a double edged sword; a change in pathophysiology leads to many adverse affects which needs active attention throughout both for prevention and management. With advancing technology, CPB continues to develop. Advances such as heparin bonded circuits, percutaneous applications of bypass, minimizing systemic inflammatory response, continued improvements in oxygenators and ventricular assist devices; all these are constantly making the conduct of CPB sophisticated and safe.
References
- Yuan SM, Shinfeld A, Raanani E. Cardio pulmonary bypass as an adjunct for the noncardiac surgeon. J Cardiovasc Med (Hagerstown) 2008; 9:338-55.
- Barry AE, Chaney MA, London MJ. Anesthetic Management during Cardiopulmonary Bypass: A Systematic Review. Anesth Analg 2015; 120: 749–69.
- Clowes GHA., Jr. Bypass of the heart and lungs with an extracorporeal circulation. In: Gibbon JH, Sabiston DC, Spencer FC, editors. Surgery of the chest. 2nd ed. Philadelphia: Saunders; 1969.
- Hessel EA 2nd. A Brief History of Cardio pulmonary Bypass Semin Cardiothorac Vasc Anesth 2014; 18:87-100.
- Passaroni AC, Silva MA de M, Yoshida WB. Cardiopulmonary bypass: development of John Gibbon’s heart-lung machine. Rev Bras Cir Cardiovasc 2015; 30:235-45.
- Machin D, Allsager C. Principles of cardio pulmonary bypass. Contin Educ Anaesth Crit Care Pain 2006; 6:176–81.
- Wallace A. Cardiovascular Disease. In: Miller RD, Pardo Jr MC (editors) Basics of Anesthesia 6th edition. Philadelphia: Elsevier; 2011: pp383-417.
- Bechtel A, Huffmyer J. Anesthetic Management for Cardiopulmonary Bypass: Update for 2014. Semin Cardiothorac Vasc Anesth 2014; 18:101-16.
- Newman MF, Croughwell ND, White WD, Lowry E, Baldwin BI, Clements FM, et al. Effect of perfusion pressure on cerebral blood flow during normothermic cardiopulmonary bypass. Circulation 1996; 94(9 Suppl): II353-7.
- Mora-Mangano C, Chow JL, Kanevsky M. Cardiopulmonary bypass and the anaesthesiologist. In: Kaplan JA (editor) Essentials of Cardiac Anesthesia 1st ed. Philadelhia: Elseviere; 2008: pp513-45.
- Stone JG, Young WL, Smith CR, et al: Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology 1995; 82:344.
- Baraka A. Alpha-stat vs. pH-stat strategy during hypothermic cardiopulmonary bypass. Middle East J Anaesthesiol 2004; 17:705-12.
- Murkin, J.M, Martzke J.S, Buchan, A.M, Bentely C, Wong CJ. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. Neurologic and cognitive outcomes. Ournal of Thoracic and Cardiovascular Surgery; 1995;110: 349-62.
- Kiziltan HT, Baltali M, Bilen A, Seydaoglu G, Incesoz M, Tasdelen A, et al. Comparison of alpha-stat and pH-stat cardiopulmonary bypass in relation to jugular venous oxygen saturation and cerebral glucose-oxygen utilization. Anesth Analg 2003; 96:644-50.
- Sakamoto T, Kurosawa H, Shin'oka T, Aoki M, Isomatsu Y. The influence of pH strategy on cerebral and collateral circulation during hypothermic cardiopulmonary bypass in cyanotic patients with heart disease: results of a randomized trial and real-time monitoring. J Thorac Cardiovasc Surg 2004; 127:12-9.
- Practice advisory for intraoperative awareness and brain function monitoring: a report by the American Society of Anesthesiologists Task Force on intraoperative awareness. Anesthesiology 2006; 104:847–64.
- Heier T, Caldwell JE. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology 2006; 104:1070–80.