Information
Journal Policies
Comparison of the Hemodynamic Response in General Anesthesia between Patients Submitted to Skin Incision with Scalpel and CO2 Laser Using Dogs as an Animal Model. A Preliminary Study
Miguel Carreira L1,Ramalho R2,Nielsen S3,Azevedo P4
2.Interdisciplinary Centre Research Animal Health (CIISA), FMV/ULisboa, Portugal.
3.Anjos of Assis Veterinary Medicine Centre (CMVAA), Barreiro, Portugal.
4.Aesculight - Woodinville, United States of America.
Copyright : © 2017 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Objectives: The study based on dog model animal, aims to evaluate if there are differences in the hemodynamic responses between patients submitted to skin incision with a blade scalpel and with a CO2 laser, using measurement changes in heart rate (HR) and arterial blood pressure (ABP) (including systolic-SAP, diastolic-DAP, and median-MAP) associated with different patient pain level perceptions during surgical procedures.
Methods: A sample of 50 dogs (N=50) of both genders, aged between one and five years, with no cardiovascular diseases, but with a soft tissue surgical clinical condition was used. The sample was divided into two groups each comprising 25 individuals. One group had midline skin incisions made with a scalpel (GS), and the other had midline skin incisions made with a CO2 laser (GL), specifically an Aesculight®. The study design considered only one surgical time point, T1 (midline skin incision), at which HR and ABP-SAP, DAP, and MAP-were measured using a high definition oscillometry (HDO) device, which allowed a fast and accurate read of the parameters. For statistical analysis, P-values < 0.05 were considered significant.
Results: All the patients presented the same pattern variation for the HR and ABP, with lower values being recorded for patients which had the midline skin incision made with the CO2-laser. The variations between GL and GS were statistically significant with a value of P< 0.001 for all the ABP parameters, but not for HR (P=0.12).
Conclusions and Relevance: Our results indicate that the use of CO2 laser in surgery surpasses the conventional scalpel, by lowering the nociceptive system stimulation, decreasing the autonomic nervous system activity and stabilizing the hemodynamic clinical signs such as the SAP, DAP, and MAP, which in turn promote reduced anesthetic consumption and thus offer greater safety to the patient.
Dog; Surgery; CO2 Laser; Scalpel; Anaesthesia; Pain,Anesthesiology
In general anesthesia, the autonomic nervous system function is affected by two main factors: surgery (type, duration, associated pain level), and the anesthetic and analgesic drugs (type and dose) which induce function variations in multiple body systems, including the cardiovascular system. As a result, changes in the heart rate (HR) and arterial blood pressure (ABP)-the systolic (SAP), diastolic (DAP) and median (MAP) parameters-can be registered [1-4]. The association between ABP and pain level is widely recognized, presenting a pattern that can be translated as-an increase in patient pain level perception corresponds to an increase in ABP values [5-8]. This relationship is the result of an interaction between the cardiovascular and nociceptive regulatory systems, mainly involving the neuroendocrine system. The hypothalamic-pituitary-adrenal axis is particularly involved with the release of ?-endorphin, adrenocortico tropic hormone, and prolactin from the anterior pituitary gland, glucocorticoids and epinephrine from the cortex and medulla adrenal glands, and norepinephrine from the sympathetic nerves.[9 - 12]
The pain phenomenon plays an adaptive role in the body, and it is the result of activation of the nociceptive system caused by a noxious stimulus detected by the nociceptors. [13,14] Nociceptors are the free nerve endings of specialized peripheral sensory neurons. [15] They are responsible for the perception and transducing of noxious external stimuli, initiating thereby the pain perception process headed by the higher levels of the central nervous system (CNS).
The skin presents assorted types of cutaneous nociceptors. [14 - 20] Different techniques of surgery are associated with different pain level perceptions by the patient. It has been postulated that carbon dioxide (CO2) laser surgery results in less post-operative pain than scalpel surgery. [21-24] The CO2 laser is one of the most widely used, with a broad range of clinical applications. It delivers a 10.600 nanometer (nm) wavelength infrared light beam which has multiple effects on soft tissues and presents only the water as a chromophore. When light energy is absorbed by water-containing tissue, tissue vaporization occurs. [23 - 28]
The study based on dog model animal, aims to evaluate if there are differences in the hemodynamic responses between dogs submitted to midline skin incision with a blade scalpel and with a CO2 laser, using measurement changes in HR and ABP (SAP, DAP, and MAP) associated with different patient pain level perceptions during surgical procedure.
2. Materials And Methods
The study was conducted on a sample of 50 regular veterinary hospital dogs (N=50) of both genders, aged between one and five years, with no cardiovascular diseases, submitted to soft tissue surgery to correct their clinical condition. These inclusion criteria allowed us to reduce the study bias variations. At no time were these animals used as experimental animals. The sample was divided into two groups each comprising 25 individuals. One group had midline skin incisions made with a scalpel (GS), and the other had midline skin incisions made with a surgical CO2 laser (GL), specifically from Aesculight®.
The study design considered only one surgical time point, T1 (midline skin incision), at which HR and ABP-SAP, DAP, and MAP-were measured using a HDO device, which allowed a fast and accurate read of the parameters [29 - 33]. All ABP measurements were taken at the left metacarpal artery after we placed a cuff with a 40-50% width of the limb circumference.
Midline skin incision and HR and ABP measurements were made only after the patient achieved an adequate and stable anaesthetic plane, characterized by the presence of Hypnosis (achieved with loss of the righting reflex) and immobility (with suppression of movement in response to the first noxious stimulus applied to the dog´s skin: the Backhaus towel clamps to fixed surgical drapes).
All the patients were submitted to a continuous intravenous administration of 0.9% NaCl fluids using a rate of 5ml/kg/hour. Anaesthesia and analgesia protocols were the same for all the patients. Prior to surgery, diazepam (0.1mg/kg), buprenorphine (0.005mg/kg), and carprofen (4mg/kg) were used. Anaesthesia was induced with propofol 1% (4-6mg/kg) and maintained with isoflurane (1.5%) administered with a medium oxygen flow rate (20 - 40 ml/kg/min) through a rebreathing system.[34] Anesthesia and surgical procedures on all patients were performed by the same anesthesiologist and surgeon.
For statistical analysis, we used SPSS® Statistics version 11.1. The Shapiro-Wilk test was used to assess the normality of the sample. Considering that all the variables presented a normal distribution we used the two-sample T-test to compare differences between groups. The results were considered statistically significant at a value of P<0.05.
3. Results
Sample characterization regarding the age, body-weight, gender, breed, and measurements of the parameters associated with hemodynamic responses, such as HR and ABP (SAP, DAP and MAP) are registered at Table 1. According to the Shapiro-Wilk test all data presented normality in both groups for the following parameters HR (P=0.97 in GS and P=0.91 in GL), SAP (P=0.91 in GS and P=0.91 in GL), DAP (P=0.91 in GS and P=0.96 in GL), and MAP (P=0.88 in GS and P=0.91 in GL) (Table 1).
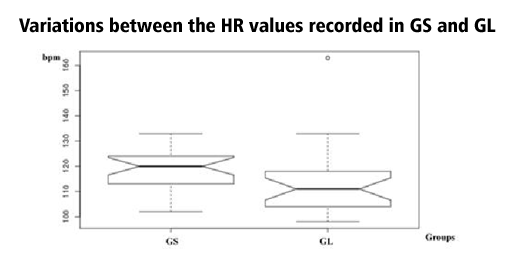
The lowest HR values were recorded in GL with a mean of 112.95 ±5.66 repeats per minute (rpm), about 4.4% less than the rpm registered in the GS. The t-test showed no statistically significant differences between both groups (P=0.12) (Table 2, Figure 1). Contrary, statistical significant differences were registered between both groups for the SAP, DAP, and MAP measurements.
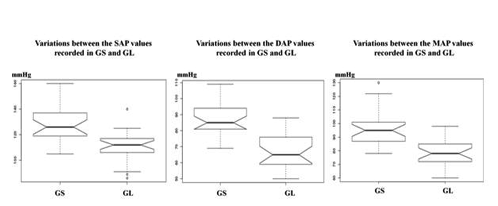
According to the results it was possible to verify that GL-SAP data was 14.9% lower than the GS-SAP; GL-DAP values were 23.2% lower than the GS-DAP; and GL-MAP values were 19.62% lower than GS-MAP, being all these variations statistically significant (P< 0.001 for all - SAP, DAP, MAP)(Table 2, Figure 2)
In this study we compared in an animal model, measurement changes in HR and ABP (SAP, DAP, and MAP), between dogs submitted to midline skin incision with a blade scalpel and a surgical CO2 laser, specifically from Aesculight®.
CO2 laser major hazards include flammability hazards such as endotracheal tube and drapes fire; gases explosion; tissue burns; ocular damage; reflection (due to the contact with specular surfaces and materials) and airborne contaminants generation resulting from the surgical plume. Thus, identifying, understanding, and controlling these major hazards and risks associated with the interaction of the laser energy with tissues or materials, is mandatory to surgeons who works with laser devices. A solid knowledge in surgical laser science allows promoting surgeons and patient safety [35].
Hemodynamic responses measured by changes in parameters such as HR and ABP are the result of the autonomic response to nociceptive stimuli. These parameters can be continuously evaluated in the anesthetized patient, and are helpful indicators of individual pain perception which is recognized by an increase in ABP values. [5-8,29,30]
The ABP indicates the extent of tissue perfusion and acts as an indirect indicator of several cardiovascular parameters. In this study, an HDO device was used to measure the ABP parameters because it was easy to use and meets the accuracy requirements of the American College of Veterinary Internal Medicine (ACVIM) and the Association for the Advancement of Medical Instrumentation (AAMI) [29 - 33].
The relationship between the cardiovascular and pain regulatory systems can be explained by the following: higher pain level perception is associated with raised ABP parameters [5,8,9,36,37].
It is known that, during the first hour of general anesthesia, variations in the hemodynamic responses recorded by the HR and ABP measurements are directly related to the type of noxious stimuli – which in the present study was the midline skin incision - and not to the isoflurane concentration that is administered to the patient [2].
According to the study results, all the patients presented the same pattern variation for the HR and ABP, with lower values being recorded for patients which had the midline skin incision made with the CO2 laser. The variations between GL and GS were statistically significant with a value of P< 0.001 for all the ABP parameters, but not for HR (P=0.12).
All patients from both groups (GS and GL) received the same analgesic protocol, including buprenorphine to prevent sympathetic stimulation and hemodynamic responses. Thus, it is reasonable to attribute the observed changes in the HR, SAP, DAP and MAP parameters to the technique used to incise the skin, which may have some effects on autonomic nervous system activity, influencing therefore the hemodynamic responses in patient and the measured cardiovascular parameters. [1,3-5]
The scalpel use is associated with high extravasations of blood. In contrast, the CO2 laser by sealing blood vessels, lymphatics, and nerve endings, reduces to a minimum the local inflammatory response, which is related to lower levels of glucocorticoids, epinephrine and norepinephrine, and therefore to lower pain level.[5,8,9,21,22,27]
Considering that skin is one of the tissues where the nociceptive system is most figurative, it is to be expected that the CO2 laser's ability to reduce stimulation of the skin nociceptive system may also be associated with a lower autonomic nervous system stimulation of the remaining tissues and organs to be operated upon, resulting therefore in discrete hemodynamic variations as registered in our study.
Thus, the lower ABP values recorded in the GL results in a decrease of the cardiac output that led to slower removal of the anesthetic from the pulmonary alveoli, because of reduced blood flow. Consequently, the anesthetic stays in the alveoli longer which reduces drug consumption during surgery, promoting the patient safety and resulting in more economical surgery. [38,39]
5. Conclusion
It is accepted that during surgical stimulation an increase in HR and ABP values is generally associated with an inadequate depth of stages and plans of general anesthesia, demanding more anesthetic consumption by the patient in order to inhibit those response.[40] By using the dog as a model, our results indicate that performing midline skin incision with the CO2 laser is associated with a lower ABP values - SAP, DAP, MAP - than with blade scalpel. This may be explain by a decrease of the nociceptive system stimulation, thus decreasing the pain and the autonomic nervous system activity, and stabilizing the hemodynamic clinical signs, such as, the ABP data.
The clinical implications of this study are significant in the context of judging the patient depth anesthetic stages and plans based on hemodynamic responses. The possibility of using CO2 laser during surgical procedures to achieve lower mean HR and ABP values appears to be important, contributing to a reduction in anesthetic consumption, decreasing their undesirable side effects and thus promoting a greater safety to the patient (human and animal).
Acknowledgments
The authors thank to CIISA - Interdisciplinary Centre of Research in Animal Health, of Faculty of Veterinary Medicine of Lisbon, of the University of Lisbon - Portugal (FMV-ULisboa); to Aesculight for the access to Aesculight CO2 surgical laser, and to Anjos of Assis Veterinary Medicine Centre (CMVAA), Barreiro - Portugal.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Filipa Mira, Alexandra Costa, Eva Mendes, Pedro Azevedo and L.Miguel Carreira. (2016). A pilot study exploring the effects of musical genres on the depth of general anaesthesia assessed by haemodynamic responses. Journal of Feline Medicine and Surgery. 18(8): 673-678. DOI:10.1177/1098612X15588968
- Cullen DJ. (1981). Drugs and anesthetic depth. In: Smith NT, Miller RD, Corbascio AN (eds): Drug Interactions in Anesthesia. Philadelphia, Lea & Febiger,:287.
- Stoelting RK, Longnecker DE, Eger EI II. (1970). Minimum alveolar concentrations in man on awakening from methoxyflurane, halothane, ether and fluroxene anesthesia: MAC awake. Anesthesiology,33:5
- Zbinden AM, Petersen-Felix S, Thomson DA. (1994). Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen anesthesia. II. Hemodynamic responses. Anesthesiology,80:261
- Saccò M, Meschi M, Regolisti G, Detrenis S, Bianchi L, Bertorelli M, Pioli S, Magnano A, Spagnoli F, Giuri P, Fiaccadori E, Caiazza A. (2013). The Relationship Between Blood Pressure and Pain. JCH, 15 (8): 600-605. DOI:10. 1111/jch.12145
- Olsen RB, Bruhel S, Nielsen CS, et al. (2013). Hypertension prevalence and diminished blood pressure-related hypoalgesia in individuals reporting chronic pain in a general population: the Tromso study. Pain; 154:257-262.
- Nordin M, Fagius J. (1995) Effect of noxious stimulation on sympathetic vasoconstrictor outflow to human muscles. J Physiol.; 489:885-894.
- Maixner W, Gracely RH, Zuniga JR, et al. (1990). Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol.;259:1156-1163.
- France CR, Froese SA, Stewart JC. (2002) Altered central nervous system processing of noxious stimuli contributes to decreased nociceptive responding in individuals at risk for hypertension. Pain.; 98:101-108.
- Campbell TS, Ditto B, Seguin JR, et al. (2003) Adolescent pain sensitivity is associated with cardiac autonomic function and blood pressure over 8 years. Hypertension; 41:1228-1233.
- Randich A, Maixner W. (1984) Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev.;8:343-367.
- Stewart KM, France CR. (1996) Resting systolic blood pressure, parental history of hypertension, and sensitivity to noxious stimuli. Pain.; 68:369-374.
- L.Miguel Carreira, Pedro Azevedo. (2016) The influence of estrogen on sex-related differences in pain perception using dog as an animal model. Glob Anesth Perioper Med; 2(3):194-199. DOI:10.15761/GAPM.1000152
- Dubin, A. E., & Patapoutian, A. (2010). Nociceptors: the sensors of the pain pathway. The Journal of Clinical Investigation, 120(11):3760-3772. http://doi.org/10.1172/JCI42843
- Dafny, N. (1997). Chapter 6: Pain Principles. Department of Neurobiology and Anatomy, University of Texas Medical School, Houston. Accessed at: [email protected]
- Foulkes T, Wood JN. (2008). Pain genes. PLoS Genet.; 4(7):e1000086. DOI: 10.1371/journal. pgen.1000086
- Raja SN, Meyer RA, Campbell JN. (1988) Peripheral mechanisms of somatic pain. Anesthesiology.;68(4):571-590. DOI:10.1097/ 00000542-198804000-00016.
- Mense S, et al. (2008) Anatomy of nociceptors. In: Bushnell MC, Smith DV, Beauchamp GK, Firestei SJ, eds.The Senses: A Comprehensive Reference. New York, New York, USA: Academic Press:11-41.
- Andrew D, Greenspan JD. (1999) Peripheral coding of tonic mechanical cutaneous pain: comparison of nociceptor activity in rat and human psychophysics. J Neurophysiol. 82 (5):2641-2648.
- Lewin GR, Moshourab R. (2004) Mechanosensation and pain. J Neurobiol.; 61(1):30-44. DOI:10.1002/neu.20078
- Miguel Carreira L., Pedro Azevedo. (2016) Comparison of the Influence of CO2-laser and Scalpel Skin Incisions on the Surgical Wound Healing Process. ARC Journal of Anesthesiology, 1(3):1-8. DOI: http:// dx.doi. org/10.20431/2455-9792.0103001
- Tambuwala, A., Sangle, A., Khan, A., & Sayed, A. (2014). Excision of Oral Leukoplakia by CO2 Lasers Versus Traditional Scalpel: A Comparative Study. Journal of Maxillofacial & Oral Surgery, 13(3):320-327. http://doi.org/ 10.1007/s12663-013-0519-2
- Bolt L. (2003) introduction to laser surgery. AAFP Proc Fall. Accessed at: vin.com/ members/proceedings/proceedings.plx
- Holmberg DL, Brisson BA. (2006) A prospective comparison of postoperative morbidity associated with the use of scalpel blades and the lasers for onychectomy in cats. Can Vet J, 47:162-163.
- Godbold JC JR. (2005). Laser lab (basic). Proc western vet conf 2005. Accessed at: Vin.com/ members/proceedings/proceedings.pl.
- Kronberger C. (2002). The Veterinary Technician's Role In Laser Surgery. Vet Clin North Am Small Anim Pract 32(3):723-735.
- Omi, T., & Numano, K. (2014). The Role of the CO2 Laser and Fractional CO2 Laser. In: Dermatology. Laser Therapy, 23(1):49-60.
- Krupa Shankar, D., Chakravarthi, M., & Shilpakar, R. (2009). Carbon Dioxide Laser Guidelines. Journal of Cutaneous and Aesthetic Surgery, 2(2):72-80. http://doi.org/10.4103/ 0974-2077.58519
- Machon R. (2012). Anaesthetic Monitoring Devices. The Vet Education Online, Veterinary Conference 2012, Vet Education Pty practice 27:512-521.
- Serpell M. (2006) Anatomy, physiology and pharmacology of pain. Surgery 24 (10): 350-353.
- Flaherty D. & Musk G. (2005). Anaesthetic monitoring equipment for small animals. In: Jr. Zollinger, Robert M; E. Christopher Ellison. Zollinger's Atlas of Surgical Operations, 9th Edition. McGraw-Hill Professional, 2010
- Martel E, Egner B, Brown SA, et al. (2013). High-definition oscillometry and direct arterial blood pressure measurement. J Feline Med Surg, Dec; 15(12):1169-70.
- Petric A, Petra Z, Jerneja S, ALenka A. (2010). Comparasion of high definition oscillometric and Doppler ultrasonic devices for measure blood pressure in anaesthaesied cats. Journal of Feline Medicine and Surgery, 12:731-737.
- Lee L. (2009). Anesthetic equipment: Breathing Circuits & Scavenging System. Veterinary Surgery I, Oklahoma State University, VMED 7412: 1-11. Accessed at: http://hallowell.com/media/09Breathing_circuit s.pdf
- Smalley, PJ. (2011). Laser safety: Risks, hazards, and control measures. Laser Therapy, 20(2):95-106.Doi:http://doi.org/10.5978/islsm.20.95
- Dworkin BR, Filewich RJ, Miller NE, et al. (1979) Baroreceptor activation reduces reactivity to noxious stimulation: implications for hypertension. Science.; 205:1299-1301.
- Saavedra JM. (1981) Naloxone reversible decrease in pain sensitivity in young and adult spontaneously hypertensive rats. Brain Res.; 209:245-249.
- Duggappa, DR., Rao, GV., Kannan, S. (2015). Anaesthesia for patient with chronic obstructive pulmonary disease. Indian Journal of Anaesthesia, 59(9):574-583. http://doi.org/ 10.4103/0019-5049.165859
- Davison R., Cottle D.(2010). The effects of anaesthesia on respiratory function. North West Deanery School of Anaesthesia, tutorial of the anaesthesia week, 15th November:1-8. Accessed at http://www.frca.co.uk/ Documents/ 205%20The%20effects%20of%20anaesthesia% 20on%20respiratory%20function.pdf
- Menon, V & Levitin, D J. (2005). The rewards of music listening: response and physiological connectivity of the mesolimbic system. NeuroImage, 28:175-184.