Information
Journal Policies
Anesthetic Management in Glaucoma Surgery
Özgün Melike Totuk Gedar1, Dilek Erdoğan Ari2, Ümit Aykan3
2 Assoc Prof, Fatih Sultan Mehmet Educational And Research Hospital Department of Anesthesiology And Reanimation, Istanbul-Turkey
3 Prof, Etiler Dünya Göz Hospital, Department of Ophtalmology, Istanbul-Turkey
Copyright : © 2016 Özgün MTG. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Elevated intraocular pressure (IOP) causes structural changes in the optic nerve head in glaucoma patients. IOP reduction is clearly the only available treatment that has been proved to decrease both the risk of disease onset and its progression. Glaucoma surgery is indicated when maximal tolerated medical or laser therapy has failed to lower IOP. Anesthesia techniques for glaucoma surgery include general, retrobulbar, peribulbar, sub-Tenon’s, subconjunctival, and topical anesthesia. General anesthesia may be advantageous for glaucoma surgery by avoiding the risks of regional anesthesia besides the total immobility of the patient, possibility to control the intraocular pressure, and the facility to fixate the operated eye. Anesthesiologists should be aware of the systemic side-effects and drug interactions of topical or systemic glaucoma medications. In addition, the effects of the anesthetic manoeuvers and drugs on IOP must be taken into consideration.
Keywords: Anesthesia; Glaucoma; Intraocular Pressure
1.Introduction
Glaucoma is a chronic and progressive group of optic neuropathies which are the leading cause of irreversible blindness in the world affecting more than 60 million people globally [1,2]. Elevated intraocular pressure (IOP) is considered to be one of the key factors in glaucoma [3]. Elevated IOP causes structural changes in the optic nerve head (ONH), including neuroretinal rim loss, cupping or excavation of the optic disc, sectoral retinal nerve fiber layer (RNFL) thinning, optic disc haemorrhage, and periapillary atrophy indicating the pathological progression of the glaucoma within whole retina [3,4]. Commonly, glaucomas are characterised by progressive gradual damage to the optic nerve and with early diagnosis most cases can be controlled well with treatment, thereby preserving vision. There are no visual symptoms in the early stages. By the years as glaucoma progresses the patient notices visual changes. Visual loss due to glaucoma can only be prevented with treatment, not reversed [5].
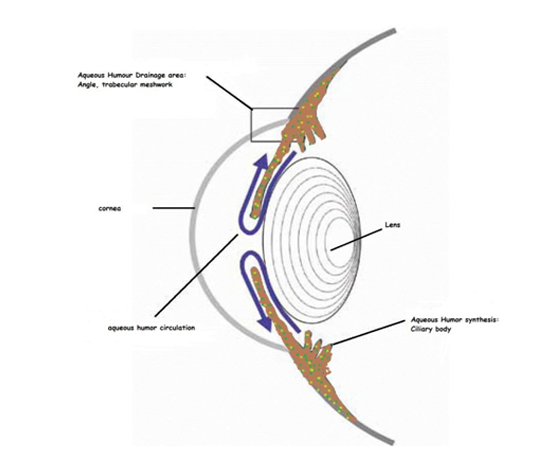
The level of IOP results from dynamic balance between the rate of aqueous humor (AH) production and its drainage. AH is produced by the nonpigmented epithelium of the ciliary bodies by diffusion, ultrafiltration, and active secretion mechanisms. Then AH flows from the posterior chamber through the pupil, fills the anterior chamber. AH leaves the eye through the trabecular meshwork (TM) into Schlemm's canal and aqueous veins (the conventional outflow pathway) and through the ciliary muscle and other downstream tissues (the uveoscleral or nonconventional outflow pathway) by passive flows.[6](Figure 1).
Glaucoma classification is quite complex because of its multifactorial nature with many different phenotypes and etiologies. According to anatomy of the anterior chamber and the drainage pathway(iridocorneal angle) glaucomas may be open-angle(OA), narrow to closed-angle (CA), and with goniodysgenesis. According to etiology, glaucoma is categorized as primary or secondary, based on several combined pathologies including comorbid conditions such as infection, mechanical injury,neovascularization, or congenital. Glaucomas can beclassified as early noncongestive, acutecongestive, and chronic with the stage of disease [7]. In addition, primary open-angle glaucoma(POAG)can present with high and low IOPs. Although IOP is normal, the typical glaucomatous axonal loss occurs, termed "normal tension glaucoma (NTG)" representing 40% of patients withPOAG. NTG patients have poor blood perfusion of the optic nerve head [3]. Primary open-angle glaucoma is the commonest type of glaucomas in European and African patients, narrow/closed angle glaucomas are more common in east Asian populations.[8]. While glaucoma can occur at any age, the vast majority of glaucoma patients are aged over 40 years [2].
IOP reduction is clearly the only available treatment that has been proved to decrease both the risk of disease onset and its progression [5]. Severalmulticenteral randomized clinical trials have consistently demonstrated that lowering IOP significantly delay or prevent glaucomatous optic nerve and visual field loss in patients with advanced glaucoma, newly diagnosed glaucoma, high IOP but no glaucoma, and glaucoma starting with lower IOP [5,9].
Commonly used medical treatments for glaucoma are topical or oral agents that decrease IOP by reducing AH productionand/or by improving the drainage through the TM and/or enhancing uveoscleral outflow [10,11].
Other methods than medical treatmentto decrease IOP include argon laser trabeculoplasty (ALT), selective laser trabeculoplasty (SLT), laser peripheral iridotomy (LPI), laser trabeculoplasty, incisional surgery (such as trabeculectomy, micro‐invasive glaucoma surgery (MIGS)) and implantation of a glaucoma drainage device(GDD), and cyclodestruction, or cycloablation [10,11.
Glaucoma surgery is indicated when maximal tolerated medical or laser therapy has failed to lower IOP to the desired target level or to control disease progression documented or anticipated; when there is intolerance or poor compliance due to a heavierexposure to topical drugs and preservatives, in terms of number of preparations and duration of medical treatment. Common surgical procedures in glaucoma are traditional incisional surgery, which includes trabeculectomy or aqueous tube shunts [12,16] Trabeculectomy with or without combined with 5-fluorouracil or with mitomycin C has been accepted as the gold standard surgery in advanced or progressing glaucoma worldwide since its introduction in the 1960s. Trabeculectomy is an open procedure, lowers IOP by making a small hole in the sclera, covered by a thin trap-door in the sclera. AH drains through the trap-door to a bleb just under the eye surface bypassing TM. [12-16]
2. Factors Affecting IOP During Surgery
The major factors controlling IOP during surgery are the dynamic balance between AH production and AH outflow; the autoregulation and chemical control of choridal blood volume; the extraocular muscle tone and vitreous humour volume [17,18].
The primary physiological control mechanism regulating IOP is balance between AH flow and AH outflow. In normal eyes, mean aqueous humor flow during waking hours has been reported to range from 2.2 to 3.1 β /min and reduces with age by 30% during a lifetime. The rates of AH flow shows circadian rhythm partly because of varying levels of catecholamines [17,18].
Epinephrine,norepinephrine,cholinergic agonist pilocarpine,physostigmine,adrenergic agonists.[17,18]
β-blockers(propranolol,timolol,betaxolol), β-agonists (clonidine,brimonidine),carbonic anhydrase inhibitors (CAI) (acetazolamide, dorzolamide, brinzolamide) [17,18]. AH outflow is relatively independent of IOP and rate limiting step is flow through the ciliary muscle in unconventional pathway, whereas is dependent of IOP and rate limiting step is flow through the inner wall of schlemm‟s canal in the conventional pathway [17,18.
Reduction in drainage Corticosteroids, age[17,18]
Direct and indirect acting muscarinic cholinergic agonists (pilocarpine, atropine, oxotremorine), adrenergic agonists, prostoglandin F2. [17,18].
The first branch of the internal carotid artery known as ophthalmic artery primarily supplies blood to the eye. The central retinal vein (CRV) solely provides the venous drainage of the anterior optic nerve. The CRV obstruction and/or increased central venous pressure associated with anxiety, restlessness, bladder fullness, coughing, retching, vomiting, Valsalva manoeuvre or straining on an endotracheal tube cause a sudden and an abrupt rise in choroidal blood volume and intraocular pressure.[17-20 Deterioration of ocular blood flow (OBF) and vascular autoregulation in the retinal, choroidal, and retrobulbar circulations are among possible mechanisms that cause retinal ganglion cell damage in glaucoma. The retinal and uveal systems are two different systems supplying blood to the eye, each having two distinct control systems: extrinsic and intrinsic. The extrinsic control system is consisted of the hormones and autonomic nervous system stimulation which maintains the systemic blood pressure (SBF) mainly controls the choroidal blood flow. The intrinsic control system involves local mediators produced by the tissues near the vessels. There are various stimulants for the retinal blood autoregulation such as changed blood oxygen level causing capillary recruitment or derecruitment providing constant oxygen supply for the tissues. Auto-regulation is impaired in glaucoma [17-20]. Ocular perfusion pressure (OPP) is defined as the mean arterial pressure (at eye level) minus the IOP. However, evidence for the potential of increasing blood pressure as a treatment for glaucoma is currently lacking, and research is hindered by the limitations of current methods to measure OBF, which lack accuracy, reproducibility, and clinical relevance.,[17-20] OPP is directly proportional to OBF and OPP fluctuation caused by changes in either systemic blood pressure or IOP may change retinal and optic nerve head perfusion [17-20].
Wang et al21 showed that optic nerve head blood flow is more susceptible to an ocular perfusion pressure decrease induced by lowering the blood pressure compared with that induced by increasing the intraocular pressure. Thus, the anesthesist should consider to protect the ocular perfusion pressure by avoiding hypotension.
Respiratory alkalosis (hyperventilation and hyperoxia), metabolic acidosis (diabetic acidosis and coma, severe muscular exercise, acidosis induced by intravenous administration of HCl or acetazolamide) can cause a decrease in IOP in both normal subjects and glaucoma patients[22,23]. On the other hand, respiratory acidosis (an increase of end-tidal CO2 in a closed-circuit rebreathing system, hypoventilation and hypercapnia) and metabolic alkalosis (intravenous administration of sodium bicarbonate) increases IOP [24].
Decrease in vitreous volume due to hyperosmotic agents (e.g.mannitol) decreases IOP. In general, a transient increase in IOP occurs following intravitreal injections.[25]
General anaesthetic agents decreases IOP directly through relaxation of extraocular muscles from central diencephalic control centres and indirectly through facilitation of aqueous drainage by hormonal and haemodynamic effects.[26].
3.Anesthesia Techniques For Glaucoma Surgery
Anesthesia techniques for glaucoma surgery include general, retrobulbar, peribulbar, sub-Tenon‟s, subconjunctival, and topical anesthesia. Local anesthesia (LA) is the mostly chosen procedure for glaucoma surgeries.[27]
Orbital regional and local anesthesia for ophthalmic surgery has traditionally been and continues to be performed by ophthalmologists. Orbital regional anesthesia is a conduction block. Safe and successful use of a conduction block depends on many factors but knowledge of anatomy, pharmacology and resuscitation skills are necessary prerequisites. The Joint Colleges report recommends that an anaesthetist must be present when a needle block is performed [28].
Retrobulbar/peribulbar anesthesia carries potential significant systemic and ocular complication risks that can threaten the patient‟s life and vision like ischemic optic nerve head injury following acute retrobulbar hemorrhage, globe perforation, increased orbital pressure, intravascular injection, inadverent optic-nerve trauma, and respiratory depression due to injection into the subarachnoid space.[29].
Subconjunctival and sub-Tenon‟s techniques may result in complications such as subconjunctival hemorrhage, chemosis, conjunctival buttonhole, failure of glaucoma surgery due to stimulation of conjunctival scarring, rarely orbital and retrobulbar hemorrage, orbital cellulitis and even globe perforation [29,30].
Intracameral anesthesia with diffusion of the local anesthetic into the posterior segment is a potential risk which may result in loss of vision if there is posterior capsule tear [30,31].
Topical anesthesia recquires good patient‟s cooperation, experinced surgical skills, may stresses surgeon due to insecurity of procedure, requires deeper sedation [31-33].
Irreversible visual loss without any obvious complication and any apparent cause after glaucoma surgery has been called wipe-out phenomenon. Causative mechanism of wipe-out phenomenon may be the sudden, intraoperative ocular hypotony during surgery which results in optic nerve hemorrhage and decreased OPP with microembolism in optic nerve fibers. The sheer volume of an LA injection may temporarily impair optic nervecirculation, and this effect may be prolonged if epinephrine is included in the LA mixture. Alternatively, the LA needle may cause a subclinical hematoma, causing pressure/ischemic damage which may result in visual loss [34,35].
It is possible to avoid eye movements duringsurgery using sub-tenon and subconjunctival anesthesia, by use of a corneal or superior rectus traction suture. Mostly, eye in the desired "looking down‟ position during surgery is used to the surgeon‟s advantage in a good cooperated patient and a traction suture is not required. Relatively short duration of topical anesthesics with the complicated prolonged surgery, patient cooperation becomes a concern. Some surgeons prefer total akinesia of the patient. The decision to perform best anesthesia technique for glaucoma surgery is based on patient‟s status and surgeons preference. General anesthesia does have its drawbacks, including risk, time, and availability of anaesthesiology team and facilities including emergency back-up and hospital beds [27-33].
An analysis of closed insurance claims by the American Society of Anesthesiologists found that 30% of claims for eye injuries associated with anesthesia were related to patient movement during surgery [36].
General anesthesia may be advantageous for glaucoma surgery by avoiding the risks of regional anesthesia besides the total immobility of the patient, possibility to control the intraocular pressure, and the facility to fixate the operated eye [27].
3. Patient Selection For General Anesthesia
- Patient preference/ refusal LA
- Pediatric age group
- Uncooperative patient(e.g. learning difficulties,language barrier, deafness,mentally challenged, agitated, psychiatric or behavioral disorders)
- Patient unable tokeep still (e.g. Parkinson‟s disease, dystonia, arthritis, nystagmus, tremor, intractable cough, dyspnoea, vertigo)
- Senile dementia
- Patient unable to lie flat for duration of operation
- Allergy to local anaesthetic agents
- Blindness on the unoperated eye
- Claustrophobia
- Long procedure
Patient‟s general health and medications must be considered. Elderly patients who may have associated extensive systemic and metabolic disorders may require highly skilled anesthesia team, well equipped surgical unit with the possibility of hospital admission if needed along with presence of intensive care backup [36].Some of the patients with congenital glaucoma have concomitant multisystem disorders. Glaucoma may be due to steroid or drug use at any age or may be related to retinopathy of prematurity in the newborn [37]. Previous studies and meta-analyses suggest that obstructive sleep apnea syndrome is associated with the prevalence of glaucoma [19].
5. Preoperative Evaluation
Preoperative evaluation is the most crucial step for decreasing anesthesia complications. Family history, patient‟s past medical problems, systemic conditions and medications should be elucidated. Medical records of patients with clinical examination helps to decide to laboratory testing for each patient specifically [26, 36,37].
Chronic anticoagulant/antiplatelet (AC/AP) medications have been involved in a statistically significant increase in the rate of both anesthesia related and surgery related intraoperative orpostoperative bleeding complications including hyphaema, intrableb bleeding and suprachoroidal haemorrhage which can result in failure of the filtration procedure or loss of sight in patients undergoing glaucoma surgery [38,39].
Most of the glaucoma surgeons often require discontinuation of antiplatelet or anticoagulant (AC/AP) medications prior to glaucoma surgery, although there is little information available to offer definitive guidance with regard to discontinuation or continuation of AC/AP therapy. Anaesthesiologists should be aware of discontinuation of AC/AP therapy, because of the risk of thrombosis [40]. Although the use of acetyl salisilic acid is associated with an increased risk of hyphaema following trabeculectomy, it is suggested safe to continue and there is no evidence to affect surgical outcome.[41]
Anaesthesiologists should be aware of the systemic side-effects and drug interactions of topical or systemic glaucoma medications.
(timolol, betaxolol, carteolol, levobunolol, metipranolol)spystemic effects: hypotension, bradycardia, bronchoconstriction, arrhythmia, congestive heart failure, bronchospasm.[42].
Topical CAI‟s do not have systemic side effects. But systemic CAI‟s can cause hypokalemia. Acetazolamide in higher doses induces metabolic acidosis especially in the elderly [43,44].
(phenylephrine,epinephrine,dipivefrine, brimonidine,apraclonidine)Epinephrine is the main representative of this group of agents, stimulating α1 and α2 adrenoceptors, as well as β2 adrenoceptors in the eye. The reduction of IOP during long term treatment is primarily due to an increase in flow through the trabecular meshwork [44]. Topical epinephrine can cause raise in systemic blood pressure, tachycardia, and precipitate cardiac arrhythmias in susceptible individuals [43,44]. Because of the occasional mydriatic effect the drug should not be used in patients with narrow chamber angles but only in patients with proven open angles [44]. As an α2 agonist, clonidine may result in systemic hypotension but aproclonidine has no effects on blood pressue or pulse rate. Brimonidine more is more -selective and more lipophilic than apraclonidine, hence lower concentrations can be used.44 In very young patients, brimonidine has resulted in apnea and coma; in elderly patients, lethargy and mental confusion [43-45].
(Echothiophate, demecarium bromide, pilocarpine). Echothiophate iodide and demecarium bromide irreversibly inhibit cholinesterase and are no longer in clinical use in many countries. Anaesthesiologists should be careful about respiratory or cardiovascular collapse side effects of long-acting cholinesterase inhibitors, if succinylcholine is used in anesthesia. Higher concentrations of pilocarpine can cause tachycardia, bronchospasm and sweating [43-45]. Acetylcholine (1% for intraocular irrigation) is occasionally used to prepare the eye for anterior segment surgery requiring rapid, complete miosis. Acetylcholine solution can theoretically cause resistance to non-depolarising muscle relaxants [44].
Hyperosmotic agents such as oral glycerol and intravenous mannitol are used in emergency situations including acute angle closure glaucoma and prior to surgery when rapid IOP reduction is needed and that cannot easily be provided by other antiglaucomatous medications [46] . These agents may cause significant fluid and electrolyte imbalance, initially with an expansion of blood volume which may cause haemodynamic problems and should be used with caution in frail or elderly people. Hyperosmotic agents should not be used in patients with kidney failure, congestive heart failure, and pulmonary diseases [46].
Normal tension glaucoma patients may additionally be treated with calcium channel blockers (e.g. nifedipine), or herbal extracts such as ginkgo biloba. The circulatory effects of calcium channel blockers and Ginkgo‟s prolongation effect on bleeding times must be taken into consideration.[44,45].
6. Effect of Anesthetic Drugs On İntra-Ocular Pressure
Opioids: alfentanil, fentanyl, morphine, remifentanil, sufentanil
Induction agents: thiopental, propofol, etomidate Benzodiazepines: iv diazepam
Inhalational agents: halothane, enflurane, isoflurane, sevoflurane, desflurane
Muscle relaxants: vecuronium, rocuronium, pancuronium
Miscellaneous: iv lidocaine, sublingual nifedipine
Induction agents: ketamine
Muscle relaxants: succinylcholine
Metoclopramide
Benzodiazepines: oral preparations, iv midazolam
Anticholinergics
Muscle relaxants: atracurium
Nitrous oxide
Anticholinesterases: neostigmine [44,45]
The central depressive effect on the diencephalic control of IOP, relaxing extraocular muscle tone and improving the aqueous humour outflow cause to reduce IOP in the use of most anesthetics [47].
Premedication will contribute to prevent IOP rise related to preoperative anxiety. Premedication with oral gabapentin in these elderly patients undergoing elective intraocular surgery produced intraoperative anxiolysis, decreased sedation, a modest decrease in IOPs and improved postoperative recovery compared to diazepam [48]. Anesthesia induction and maintenance must focus to inhibit IOP pressure increase, and provide haemodynamic stabilisation. Induction must be smooth, avoiding coughing and cardiovascular reflexes due to laryngoscopy and tracheal intubation. It will be appropriate to avoid the use of succinylcholine and wait for the induction drugs to have their full effect. A combination of propofol with remifentanil is reported to decrease IOP even after intubation with succinylcholine [47]. And it has been shown that anesthetic regimens with sevoflurane and remifentanil are comparable to total intravenous anesthesia with propofol and remifentanil. The maintenance of anesthesia using isoflurane in combination with remifentanil is found less effective than propofol-remifentanil combination in the management of IOP in cataract surgery [47]. Dexmedetomidine and clonidine are two alpha-2 adrenergic agonist drugs which have the property of inhibiting rise in IOP following suxamethonium, laryngoscopy, and tracheal intubation [49].
The introduction of supraglottic devices has allowed many ophthalmic surgical procedures to be performed whilst patients breathe spontaneously. [28] Muscle relaxants are not usually required with the laryngeal mask airway (LMA) if total intravenous anesthesia is used. Insertion and removal of a tracheal tube causes more cardiovascular stimulation than LMA. Breath-holding, laryngospasm, bronchospasm and coughing are less likely with LMA. But some patients who can not maintain their eyes in neutral position under total intravenous anesthesia require a small dose of muscle relaxant. If muscle relaxants must be avoided, the surgeon can return the eye to a neutral position using a traction suture [36].
Another important factor affecting intraocular hypertension is mydriasis. Mydriasis causes the normally open anterior chamber angle to close and increases intraocular pressure. Deep anesthesia induced by anesthetics at concentrations higher than that of clinical use, postoperative care in a darkened room, psychological stress, and parasympatholytic drugs (such as atropine and scopolamine) or sympathomimetic drugs (such as phenylephrine and ephedrine) have been associated with mydriasis [50]. Although high concentrations of inhalation anesthetic drugs can cause mydriasis, the concentrations of inhalation anesthetic drugs that induce mydriasis are considered to be far higher than the clinical concentrations [50]. Sympathomimetic drugs dilate the pupil and elevate blood pressure significantly. These drugs may also cause acute angle closure glaucoma (AACG) in patients with a predisposing condition. Phenylephrine can reportedly cause AACG. Some postoperative AACG cases are reportedly related to ephedrine. Despite its short vasoactive effect, ephedrine can result in dilatation of the pupil for 3–6 hours. For this reason, in cases of known and untreated AACG, ephedrine is contraindicated [50]. Although anticholinergics, given as antisialogogues, have little effect on IOP when given intramuscularly before the arrival at the operating theatre or intravenously at induction, atropine should not be used in the presence of narrow chamber angles [45]. Propofol administered during induction of anesthesia causes a significant decrease in blood pressure, which is especially important for the elderly. Ephedrine has both α and β adrenergic properties and is helpful in the treatment of hypotension, but is better to be avoided because of the possibility of AACG. Some studies have demonstrated that the co-administration of propofol and ketamine is more favourable due to the stabilisation of the haemodynamics. Although ketamine may rise or does not effect IOP, the combination of ketamine with propofol provides moderate decrease in IOP with minimal haemodynamic changes.51 Compared to dexmedetomidine, at similar sedation levels, sedation provided by ketofol (200 mg propofol and 100 mg ketamine solution) enables satisfactoryanalgesia. Moreover, ketofol has a more rapid onset of action and a shorter recovery period from anesthesia without causing significant haemodynamic or respiratory adverse effects [52]
.
Maintenance crystalloid fluids will be sufficient for intravenous fluid regimen. The clinician should consider that a full bladder causes hypertension and elevates intraocular pressure, especially during prolonged cases [36].
Prevention of postoperative nausea and vomiting is essential for the control of IOP in the postoperative period. Propofol and remifentanil are preferable drugs because of their antiemetic properties [36,53] Pre-induction administration of iv metoclopramide is reported to cause a small rise in IOP lasting about 30 minutes.45 Onsansetron, granisetron, and dexamethazone must be preferred for the prevention of nausea and vomiting.
Shivering can cause an increase in blood pressure and intraocular pressure besides many other side effects. It must be prevented by the use of dexamethazone, dexmedetomidine or classically by pethidine[54].
Extubation must be performed deep with spontaneous breathing in order to avoid coughing and straining on the tracheal tube [36]. Intravenous lidocaine administration is shown to decrease IOP and it prevents coughing during extubation[53]
Pain relief is essential and easily provided by the use of non-steroidal antiinflamatory drugs in the postoperative period [36].
In conclusion, preoperative evaluation of the glaucoma patient deserves special attention. The anesthesiologist should be aware of the systemic side-effects and drug interactions of topical or systemic glaucoma medications. Smooth intubation and extubation, prevention of IOP rise and haemodynamic instability, profilaxis of postoperative nausea and vomiting and pain relief are the main points of the anesthetic management.
References
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081-90.
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262-7.
- Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012;119(5):979-86.
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996;80:389-93.
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120:1268-79.
- Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur J Pharm Biopharm 2015;95:173-81.
- European Glaucoma Society. Terminology and Guidelines for Glaucoma. 3rd ed. Savona: Dogma; 2008.
- Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ 2004;82(11):887-88
- Kass MA, Heuer DK, Higginbotham EJ et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701-13.
- Leske MC, Wu SY, Hennis A et al. BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados eye studies. Ophthalmology 2008;115(1):85-93.
- Bagnis A, Papadia M, Scotto R, Traverso CE. Current and emerging medical therapies in the treatment of glaucoma. Expert Opin Emerg Drugs 2011;16(2):293-307.
- Edmunds B, Thompson JR, Salmon JF, Wormald RP. The national survey of trabeculectomy. II. Variations in operative technique and outcome. 2001;15:441-8.
- Edmunds B, Thompson JR, Salmon JF, Wormald RP. The national survey of trabeculectomy. III. Early and late complications. Eye (Lond) 2002;16:297-303.
- Stalmans I, Gillis I, Lafaut AS. Safe trabeculectomy technique: Long term outcome. Br J Ophthalmol 2006;90:44-7.
- Papadooulos M, Khaw PT. Improving glaucoma filtration surgery. Eye 2001;15:131-2.
- Mosaed S, Minckler DS. Aqueous shunts in the treatment of glaucoma. Expert Rev Med Devices 2010;7:661-6.
- Cunningham AJ. Intraocular pressure - physiology and implications for anaesthetic management. Can Anaesth Soc J 1986;33(2):195-208.
- Malihi M, Sit AJ. Aqueous humor dynamics and implications for clinical practice. Int Ophthalmol Clin 2011;51(3):119-39.
- Nakazawa T. Ocular blood flow and influencing factors for glaucoma. Asia Pac J Ophthalmol 2016;5(1):38-44.
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous Humor Dynamics: A Review. Open Ophthalmol J 2010;4:52-9.
- Wang L, Cull GA, Fortune B. Optic nerve head blood flow response to reduced ocular perfusion pressure by alteration of either the blood pressure or intraocular pressure. Current Eye Research 2015; 40(4):359-67.
- Gallin-Cohen PF, Podos SM, Yablonski ME. Oxygen lowers intraocular pressure. Invest Ophthalmol Vis Sci 1980;19:43–8.
- Petounis AD, Chondreli S, Valaduka-Seikoti A. Effect of hypercapnea and hyperventilation on human intraocular pressure general anesthesia following acetazolamide administration. Br J Ophthalmol. 1980;64(6): 422–5.
- Hosking SI, Evans DW, Embleton SJ et al. Hypercapnia invokes an acute loss of contrast sensitivity in untreated glaucoma patients. Br J Ophthalmol 2001;85:1352–6.
- Höhn F, Mirshahi A. Impact of injection techniques on intraocular pressure (IOP) increase after intravitreal ranibizumab application. Graefes Arch Clin Exp Ophthalmol 2010;248:1371–5.
- Gomi CF, Yates B, Kikkawa DO, Levi L, Weinreb RN, Granet DB. Effect on intraocular pressure of extraocular muscle surgery for thyroid-associated ophthalmopathy. Am J Ophthalmol 2007;144(5):654-7.
- Jaichandran VV. Anesthesia for glaucoma surgery. J Curr Glaucoma Pract. 2010;4(2):49-55
- Kumar CM, Dowd T. Evolving roles of the anaesthetist in ophthalmic surgery. Trends in Anaesthesia and Critical Care 2011;(1):176-8
- Morgan CM, Schatz H, Vine AK et al. Ocular complications associated with retrobulbar injections. Ophthalmology 1988;95:660–5.
- Kansal S, Moster MR, Gomes MC, Schimidt Jr CM, Wilson RP. Patient comfort with combined anterior sub-Tenon's, topical and intracameral anesthesia versus retrobulbar anesthesia in trabeculectomy, phacotrabeculectomy and aqueous shunt surgery. Ophthalmic Surg and Lasers 2002;33:456-62.
- Pablo LE, Ferreras A, Pérez-Oliván S, Larrosa JM, Gómez ML, Honrubia FM. Contact-topical plus intracameral lidocaine versus peribulbar anesthesia in combined surgery: A randomized clinical trial. J Glaucoma 2004;13(6)510-5.
- Geffen N, Carrillo MM, Jin Y, Trope GE, Buys YM. Effect of local anesthesia on trabeculectomy success. J Glaucoma 2008;17(8):658-61.
- Lai JS, Tham CC, Lam DS. Topical anesthesia in phacotrabeculectomy. J Glaucoma 2002;11(3):271-4
- Gracner T, Pahor D. The outcome of filtration surgery in end-stage glaucoma. Zdrav Vest 2012;81:29-36.
- Baser E, Seymenoglu G, Mayali H. Trabeculectomy for advanced glaucoma. Int Ophthalmol 2011;31:439-46
- Pritchard N. General anaesthesia for ophthalmic surgery. Anaesth Int Care Med 2004;5(9):30710.
- Lord J. Anaesthesia for eye surgery in paediatrics. Anaesth Int Care Med 2004;5(9):314-7.
- Balbino M, Boin P, Prata TS. Perioperative management of anticoagulant users scheduled for glaucoma surgery: a survey among the Brazilian Glaucoma Society members. Arq Bras Oftalmol. 2013;76(6):363-5.
- Kiire CA,Mukherjee R, Ruparelia N, Keeling D, Prendergast B, Norris JH. Managing antiplatelet and anticoagulant drugs in patients undergoing elective ophthalmic surgery. Br J Ophthalmol 2014;98:1320-4.
- Alwitry A, King AJ, Vernon SA. Anticoagulation therapy in glaucoma surgery. Graefes Arch Clin Exp Ophthalmol 2008;246(6):891-6.
- Cobb CJ, Chakrabarti S, Chadha V, Sanders R. The effect of aspirin and warfarin therapy in trabeculectomy. Eye (Lond) 2007;21(5):598-603
- Stewart WC, Castelli WP. Systemic side effects of topical beta-adrenergic blockers. Clin Cardiol 1996;19:691-7.
- Goldberg I, Moloney G, McCluskey P. Topical ophthalmic medications: what potential for systemic side effects and interactions with other medications? Med J Aust. 2008;189(7):356-7.
- Hoyng P, Van Beek L. Pharmacological therapy for glaucoma, a review. Drugs 2000;59(3): 41134
- Raw D, Mostafa SM. Drugs and the eye. Br J Anaesth 2001;1(6):161-5.
- Boey PY, Singhal S, Perera SA, Aung T. Conventional and emerging treatments in the management of acute primary angle closure. Clin Ophthalmol 2012;6: 417–24.
- Montazeri K, Dehghan A, Akbari S. Increase in intraocular pressure is less with propofol and remifentanil than isoflurane with remifentanil during cataract surgery: A randomized controlled trial. Adv Biomed Res 2015;4:55.
- Kavitha J, Parida S, Kundra P, Srinivasan R. Oral gabapetin premedication for elderly patients undergoing intraocular surgery. Br J Ophtalmol 2013; 97(7):900-4.
- Banga PK, Singh DK, Dadu S, Singh M. A comparative evaluation of the effect of intravenous dexmedetomidine and clonidine on intraocular pressure after suxamethonium and intubation. Saudi J Anesth 2015;9(2):179-83
- Nitta Y,Kamekura N, Takuma S, Fujisawa T. Acute angle-closure glaucoma after general anesthesia for bone grafting. Anesth Prog 2014;61(4):162-4.
- Aydogan MS, Demirel S, Erdoğan MA, Fırat P, Colak C, Durmuş M. Effects of ketaminepropofol mixture on intraocular pressure and haemodynamics in elderly patients: A randomised double-blind trial. Turk J Anaesth Reanim 2014;42:12-8.
- Yagan O, Karakahya RH, Taş N, Kucuk A. Katarakt cerrahisinde deksmedetomidin ve ketaminpropofol sedasyonunun karşılaştırılması. Turk J Anaesth Reanim 2015;43:84-90.
- Bor C,Certug A. Anaesthesia in ophtalmic surgery. Ege Medical Journal 2015;54(1):46-53.
- Entezariasl M, Isazahdehfar K. Dexametazone for prevention of postoperative shivering: a randomized double-blind comparison with pethidine. Int J Prev Med 2013;4(7):818-24.