Information
Journal Policies
ARC Journal of Anesthesiology
Volume-1 Issue-3, 2016, Page No: 1-8
Comparison of the Influence of CO2-laser and Scalpel Skin Incisions on the Surgical Wound Healing Process
Short Running Title: CO2-laser vs scalpel skin incisions and wound healing
L.Miguel Carreira1,2,3, Pedro Azevedo3
1.Faculty of Veterinary Medicine, Department of Clinic, Surgery, University of Lisbon (FMV/ULisboa), Av. da Universidade Técnica de Lisboa, 1300-477 Lisbon, Portugal.
2.Centre for Interdisciplinary Research in Animal Health (CIISA), FMV/ULisboa, Av. da Universidade Técnica de Lisboa, 1300-477 Lisbon, Portugal.
3.Anjos of Assis Veterinary Medicine Centre (CMVAA), Rua D.ª Francisca da Azambuja Nº9 -9A-Barreiro, Portugal
2.Centre for Interdisciplinary Research in Animal Health (CIISA), FMV/ULisboa, Av. da Universidade Técnica de Lisboa, 1300-477 Lisbon, Portugal.
3.Anjos of Assis Veterinary Medicine Centre (CMVAA), Rua D.ª Francisca da Azambuja Nº9 -9A-Barreiro, Portugal
Citation : Carreira LM, Azevedo P. Comparison of the Influence of CO2-laser and Scalpel Skin Incisions on the Surgical Wound Healing Process. ARC Journal of Anesthesiology. 2016;1(3):1–8. DOI : dx.doi.org/10.20431/2455-9792.0103001
Copyright : © 2016 Carreira LM. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Several factors influence the development of the surgical wound healing process. Conventional incisions with a scalpel are always associated with greater haemorrhaging, resulting in increased surgical time and inflammatory reaction, in contrast to CO2-laser incisions.
Objective: The study aimed to evaluate the influence of CO2-laser and scalpel skin incisions on the surgical wound healing process
Animals: We used a sample of 70 dogs (N = 70) of both genders submitted to elective spay surgeries.
Methods: The sample was divided in two groups according to the surgical method performed: group S (scalpel) and group L (CO2 laser). Assessment was made over three time points: T0 (before surgery), T1 (48 hours after surgery) and T2 (8 days after surgery). Blood samples were collected to quantify the variations in white blood cells (WBC), plasmatic protein total (PPT) and serum albumin (ALB). To rate a patient’s pain level, we used the Melbourne Pain Scale (MPS). We followed up with the patients to evaluate the wound for closure time, dehiscence and scar appearance.
Results: Skin incisions made with the CO2 laser are associated with lower WBC counts and minor tissue trauma because the endothelial wall does not incur as much injury as with scalpel incisions, thus decreasing the PPT and ALB extravasation levels and promoting the healing process. At T1, the individuals in group L had lower pain levels and consequently exhibited greater post-operative patient comfort. The cosmetic results were different between the methods, with 100% of the group L patients showing very good scar appearances eight days after surgery. Conclusion: These findings show that the method selected to perform skin incisions influences the healing process of the affected tissues.
1.Introduction
The CO2 laser is an efficient, versatile, powerful surgical tool with different applications [1,-7] . In this tool, the CO2 laser beam is directed through the internal mirror system of an articulated surgical arm, creating a focused laser beam that can be used as a thermal scalpel on tissue [1]. However, its use is limited to open surgery [6]. When the laser interacts with the tissue, the light can be absorbed, reflected, transmitted, or scattered. Depending on its wavelength, a laser will have the greatest effect on tissues’ water, haemoglobin or melanin content. The most common types of lasers operate by transforming light energy into heat to evaporate intracellular water8. The power density of a surgical laser determines whether the beam will be used to vaporize and destroy tissue or to incise or excise tissue like a scalpel. During a surgical procedure, the beam can be applied continuously or can be interrupted [1, 7]. The main advantages of using a CO2 laser instead of scalpel to make skin incisions are 1) the ability to make accurate incisions with focal points of 0.1–0.3mm; 2) the instantaneous sealing of small blood vessels, lymphatics and nerve endings; 3) decreased surgery time; 4) decreased post-operative inflammatory reaction and pain; and 5) the antiseptic effect provided when microorganisms evaporate under the beam [1, 8- 14]. The major disadvantages associated with the laser equipment include its high costs, lengthy surgeon training, and the need to equip surgical areas with supplementary devices, such as a smoke evacuator, to ensure surgeon and patient safety. The aim of this study was to evaluate whether the method used to make a surgical skin incision, either a CO2 laser or a scalpel, influences the surgical wound healing process by studying variations in white blood cells (WBC), serum plasmatic protein total (PPT), serum albumin (ALB), and patients' pain levels and by following up to assess the skin thickness, closuretime, dehiscence and scar appearance parameters of the wound itself.
2. Materials and Methods
The study was developed using a sample of 70 dogs (n = 70) of both genders. These animals were regular hospital patients submitted for elective spay surgeries involving ovariohysterectomy (OHE) in females and normal orchiectomy (OE) in males. At no time were these animals used as experimental animals, and the use of the patients in the study began only after the owners signed consent forms. The sample was divided into two groups of 35 individuals each according to the skin incision method performed: group S (incision with scalpel) and group L (incision with CO2 laser). Blood samples were collected from each patient at three time points: T0 (immediately before surgery) for routine pre- surgical analysis, T1 (48 hours after surgery) and T2 (eight days after surgery). These blood samples were used to quantify the WBC, PPT, and ALB parameters, thus obtaining an understanding of the healing process using laboratory data. The surgical wound itself was assess at time points T1 and T2, allowing us to obtain data showing inflammatory reaction signs, closure time, dehiscence, the thickness of the affected skin and scar appearance. To measure the skin thickness, we used a digital calliper. To compare the final macroscopic appearance of the scar, we used a four-level rating scale: bad (marked hypertrophy of the skin with keloid formation) = 1; regular (medium hypertrophy of the skin) = 2; good (discrete or small hypertrophy of the skin) = 3; and very good (normal skin appearance) = 4. For scalpel incisions, we used a number-10 blade; for CO2 laser incisions, we used an Aesculigth Model 1507 Surgical Laser System® (Aesculight,Woodinville,USA) with 15 watts CW and 7 watts average super pulse power. In terms of the size of the incision made, we always sought to achieve the smallest possible incision, adjusting it to each patient. The incision mean was 5.51 ± 1.50 cm for group S and 5.49 ± 1.27 cm for group L. The patient’s pain scores were evaluated with the Melbourne Pain Scale (MPS) at T1 and T2. Because the MPS scale is subjective, the assessors were blinded in their evaluation regarding to which group a particular animal belonged. At T0, all patients received a therapeutic protocol that included amoxicillin and clavulanic acid (Synulox; Zoetis, Portugal) 10 mg/kg, tramadol (Tramal; Actavis UK) 4 mg/kg, and meloxicam (Metacam; Boehringer Ingelheim Vetmedica,St. Joseph) 0.2 mg/kg. For anaesthesia induction, we used propofol (PropoFlo; Abbott Animal Health) 4 mg/kg and isoflurane (Isoflo; Laboratório Esteve Veterinária) for maintenance. Statistical analyses were conducted with the Social Science Statistics software, including the Shapiro-Wilk test for sample normality and the paired t-test to compare the groups. All results were evaluated for statistical significance at P < 0.05.
3. Results
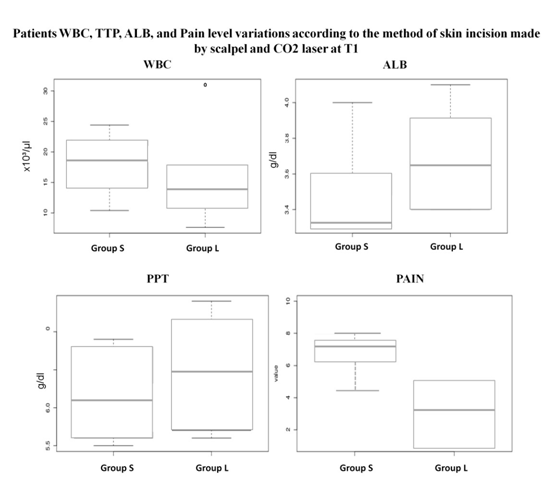
The collected data regarding the total sample, and each considered group (S and L) characterization for gender, age, body weight, incision long, breed, laboratory parameters (WBC, PPT, ALB), patients pain level and the wound evolution characterization (closure time, dehiscence, scar appearance) are compiled in Table 1. The Shapiro-Wilks test showed that sample results were presented a normally distribution for all the studied parameters. Comparisons between the groups S and L regarding the laboratory parameters, and the thickness of the skin comparison were performed at T1 and T2 using paired t-test. The other parameters associated with the follow-up of wound evolution namely the closure time and scar appearance were evaluated only at T2. For the WBC the S group had a leukocytosis 25% higher than the L group, with a mean difference between the two groups at T1 showing a statistically significant difference (P = 0.02), but not at T2 (P = 0.85) (Table 2, Figure 1). Although no statistically significant differences in PPT were registered between the two groups at T1 (P = 0.07) and T2 (P = 0.77), the S group showed lower values than the L group at both time points (Table 2, Figure 1). According to our results ALB values showed statistically significant differences between S and L groups at T1 (P = 0.001) with the L group presenting almost 13.0% higher ALB mean values than the S group, but not at T2 (P = 0.54) (Table 2, Figure 1). Subjects of groups had a significantly higher pain level values than those in the L group with a MPS final score of 7.51 comparing to the 3.28 respectively (Table 1, Figure 1). Statistically significant differences were registered between the two groups at T1 (P = 0.01), with the L patients presenting a value of 60.0% less than the S patients for pain score level. No differences were registered for this factor at T2, since none of the patients of both groups exhibited pain at that time point (Table 2). Regarding the parameters associated with the wound evolution, it was possible to notice for the parameter skin thickness that no statistically differences were registered between the S and the L groups at T1 (P = 0.34), contrary to what was registered at T2 (P = 0.00). For the other two parameters, the closure time of the wound and the scar appearance, it was possible to registered statistically significant differences between both groups at T2 (P > 0.00 for both parameters) with the L group patients presenting an earlier healing process than the S group, and all (100%) rating to the cosmetic effect of the scar as very good in contrast with the 28.5% of the S group (Table 2).
4. Discussion
The sample was uniformly distributed in both groups (S and L) regarding the age, gender, breed and type of surgical procedures to reduce the variability of results because these parameters could bias the findings. To standardize the data, the same surgeon performed all the surgical procedures and the number of OHEs and OEs included in each study group were similarly distributed because OHE is a more painful surgical procedure than normal OE. In addition, because different breeds have different responses to pain [15-17], we attempted to distribute different breeds equitably between both groups and genders. Wound healing involves interactions between cytokines, matrix components and a variety of blood components and cells [18-21]. It is divided into three overlapping phases: an inflammatory phase, a proliferative or repair phase and a mature or remodelling phase [22]. The inflammatory phase is mainly characterized by the local invasion of cytokines and WBCs to eliminate the initial cause of cell injury and initiate the tissue repair process. The WBC count is directly related to tissue injury, with each type of incision having a greater or lesser regional inflammatory response. Conventional incisions with a scalpel are always associated with greater haemorrhaging, resulting in increased surgical time and inflammatory reaction [23, 24]. In contrast, CO2 laser incisions are associated with good haemostasis, minor bleeding and decreased surgical time, thus promoting less inflammatory reaction [25-28]. Furthermore, because the CO2 laser incision can be made without having direct contact with the intervened region, this method provides a lower risk of infection and, consequently, a lower need to mobilise WBCs to the region, thus shortening the inflammatory phase and a less pronounced incision compared to one resulting from direct contact from sharp instruments. In the present study, T1 (48 hours after surgery) corresponds to the peak of the inflammatory phase, which occurs shortly after the incision and can last for up to five days [20, 23]. In addition, the WBC count was higher for both groups at T1, but still the count was lower in group L than in group S. According to the results, statistically significant differences were seen in the WBC counts between T0 and T1 in group S (P = 0.02) and group L (P = 0.01) but not between T0 and T2 in group S (P = 0.14) and group L (P = 0.27). The proliferative phase is characterized by the presence of a large number of fibroblasts, collagen production and local angiogenesis but a lower WBC number. Statistically significant differences were registered at T1 between groups S and L for the WBC count (p = 0.02), contrary to the data obtained at T2 (P = 0.85). Nevertheless, patients in group L showed lower levels of WBCs than those in group S. As a result, CO2 laser-incised tissues are more likely to pass into the second phase of wound healing, the proliferative phase, more quickly than scalpel-incised tissues. This indicates that in group S, more tissue trauma occurs, allowing higher PPT extravasation levels through the endothelium of the injured vessels than that seen in group L. The CO2 laser induces the instantaneous vaporization of cell structure with little or no release of inflammation mediators. At the same time, the denaturation of the matrix proteins, such as collagen, occurs, forming a layer on the cell surface that likely acts as an impermeable shield [29], thereby decreasing PPT loss. Because hyperproteinaemia delays wound healing due to decreased collagen synthesis, fibroblast proliferation and angiogenesis, the patients in group S theoretically have a higher risk of experiencing delayed healing than those in group L. Nevertheless, none of the patients in the groups experienced delays in healing process because no dehiscences were registered in either group. With regard to ALB, about two-thirds of the total amount of ALB is found in the extravascular compartment and only one-third in the intravascular compartment, showing that its concentration decreases in cases of tissue aggression, such as surgery30. Although ALB has a long half-life (12–21 days), thus being considered a poor indicator of the metabolic state of the patient, ALB is regularly used to infer variations in the level of acute metabolic stress that tissues experience31. During inflammation, ALB moves from circulation and intravascular spaces to the liver, thus promoting a decrease in concentrations soon after an injury, which remains until the inflammatory process is resolved [32-35]. The mechanism of the ALB’s movement into the extravascular compartment depends on the permeability of the endothelial wall and the hydrostatic and oncotic pressures on either side of it [36]. In addition, the sequestration of ALB into non‐exchangeable locals, such as wounds, is common [35,37]. In patients submitted to elective surgery or in those with critical injuries, more tissue injuries lead to higher ALB depletion, thus making the wound healing process slower and poorer [38-45]. The higher MPS pain scores in group S and the low MPS pain scores in group L at T1 are in line with some studies indicating that patients undergoing CO2 laser incisions experience a reduction in post-surgical pain. This might be a result of the laser’s thermal properties, which provide a vaporization effect as it cuts tissues and promotes the carbonization of sensory nerve endings [46-51]. We viewed the higher MPS pain scores at T1 in group S as only being associated with the direct tissue trauma itself rather than the possibility that this pain comes from the systemic inflammatory response because grievances were only associated with the direct manipulation of the affected region. Nevertheless, it is only possible to confirm this if we test the inflammatory markers and compare the obtained MPS scores to determine if they are elevated or similar to those of group L. A direct comparison of the wounds’ healing at T1 showed no differences between groups S and L in terms of the skin thickness (P = 0.34) of the affected area, contrary to what was seen at T2 (P = 0.00). In addition, by comparing the final macroscopic appearance of the scar, it was possible to determine that 100% of the patients in group L presented a scar classified as very good, which is very different from the result obtained in group S (28.5%). These results are in line with other studies that also concluded that incisions made using a CO2 laser are associated with better cosmetic appearances [49-52]. It would have been desirable to obtain and analyse small tissue biopsies from the healing incisions to conduct a microscopic study of tissues at T1 and T2. This was not possible because, as stated before, these animals were at no time used as experimental animals.
In this study, we concluded that skin incisions made using a CO2 laser are associated with lower WBC counts, therefore reducing the inflammatory response in injured tissues, allowing an earlier proliferative phase and hastening tissue healing. In addition, by inducing a minor tissue trauma the endothelial wall was injured less, allowing a decrease in the PPT and ALB extravasation levels and promoting the healing process. At T1, the individuals in group L had lower pain levels and consequently exhibited greater post-operative comfort. The cosmetic results were very different between both skin incision methods, with 100% of the patients in group L showing a very good scar appearance eight days after surgery. In conclusion, the method selected to perform skin incisions influences the healing process of affected tissues.
5.Acknowledgments
The authors thank to to the Editor of the ARC Journal of Anesthsia for his support allowing having this ARC-Free article submission; to Centre for Interdisciplinary Research in Animal Health – CIISA, of Faculty of Veterinary Medicine of Lisbon, of the University of Lisbon – Portugal (FMV-ULisboa); to Prof. Dr. João Dias for the CO2-laser device; and to Anjos of Assis Veterinary Medicine Centre (CMVAA), Barreiro – Portugal.
References
- Wright VC. Laser surgery: using the carbon dioxide laser. Can. Med. Assoc. J. 1982; 126(9): 1035-1039.
- Shankar DSK, Chakravarthi M, Shilpakar K. Carbon Dioxide Laser Guidelines. J Cutan Aesthet Surg. 2009; 2(2):72–80
- Ross HM, Smelsoytoy JA, Davis GJ. Photodynamic therapy with motexafin lutetium for retal cancer: A pre-clinical model in the dog. J. Surg Res. 2006; 323-530.
- Davidson EB, Ritchey JW, Higbee RD. 2004. Laser lithotripsy for treatment of canine uroliths.Vet Surg. 2004:56-61.
- Holt TL, Mann FA. Soft tissue application of lasers. Vet Clin North am Small Anim Pract.2002:569-599.
- Dye TL, Teague HD, Ostwald DA. Evaluation of a technique using the carbon dioxide laser for the treatment of aural hematomas. J Am Anim Hosp Assoc. 2002: 385-390.
- Ross HM, Smelsoytoy JA, Davis GJ. Photodynamic therapy with motexafin lutetium for retal cancer: A pré-clinical model in the dog. J. Sung Res. 2006: 323-530.
- Lucroy MD, Bartels KE. Using biological lasers in veterinary practice. Vet Med. 2000; 95(10): 4-9
- Durante EJ, Kriek NP. Clinical and histological comparison of tissue damage and healing following incisions with the CO2 laser and stainless steel surgical blade in dogs. J. S. Afr. Vet.Assoc.1993; 64(3):116-120
- Berger N, Eeg PH. Fundamentals of laser-tissue interactions. In:Veterinary Laser Surgery: A practical guide.1st Ed. Blackwell Publishing. 2006; 29-42.
- Berger N, Eeg PH. Laser systems, wavelengths, and technology selection. In: Veterinary Laser Surgery: A practical guide. 1st Ed. Blackwell Publishing. 2006; 63-75.
- Berger N, Eeg PH. Types of laser-tissue interaction related to the rate of heat transfer though soft tissue. In: Veterinary Laser Surgery: A practical guide.1st Ed. Blackwell Publishing. 2006; 43-61.
- Mison M.B., Bohart G.H., Walshaw R. Use of carbon dioxide laser for onychectomy in cats. J Am Vet Med Assoc. (2002) 651-653.
- Holmberg DL, Brisson BA. A prospective comparison of postoperative morbidity associated with the use of scalpel blades and lasers for onychectomy in cats. Can Vet J. 2006:162-163.
- Sharkey M. The Challenges of Assessing Osteoarthritis and Postoperative Pain in Dogs. The AAPS Journal 2013; 15(2):598-607. Doi: 10.1208/s12248-013-9467-5.
- NRC - National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and Alleviation of Pain in Laboratory Animals. Washington (DC): National Academies Press (US) (2009). Available at: http://www.ncbi.nlm.nih. gov/ books/NBK32656/
- Dobromylskyij P, Flecknell PA, Lascelles BD, et al. Pain assessment. In: Flecknell PA, Waterman-Pearson A, et al., editors. Pain management in animals. London, Saunders, 2000; 53– 79.
- Dyson M. Advances in wound healing physiology: the comparative perspetive. Veterinary Dermatology.1997; 8(4):227-233
- Liptak JM. An overview of the topical management of wounds Australin Veterinary Jounal.2013; 75(6):408-413
- Pavletic MM. Atlas of small animal wound management and reconstructive surgery. 3th ed.Wiley – Blackwell, Iowa 2010.
- Scheremi S, Szeimies RM, Karrer S, Heinlin J, Landthaler M, Babilas P. The impact of the ph value on skin integrity and cutaneous wound healing. Journal of the European Academy of Dermatology and Venereology. 2010; 24(4):373-378
- Pinto AM. Fisiopatologia: Fundamentos e Aplicações. Lisboa, Lidel, 2007.
- Hedlund CS. Surgery of the tegumentary system. In: Fossum, T.W. Small animal surgery. 3th ed.Missouri, Mosby Elsevier, 2007; 161-259.
- Gerhardt M.O. Efeitos da utilização de laser de CO2, eletrobisturi e bisturi convencional junto a estruturas nervosas periféricas. Revista Odonto Ciência. 2004; 19(45): 265-69.
- Liboon J, Funkhousor W, Terris DJ. A comparison of mucosal incisions made by scalpel. CO2 laser, eletrocautery and constant voltage eletrocautery. Oto Laryngol Head Neck Surg. 1997; 379- 385.
- Fisher J, Sheila E, Frame W, Browne M, Tranter MD. A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa.Archs oral biol. 1983;28(4):287-291
- Paggiaro AO, Teixeira Neto N, Ferreira MC. Princípios gerais do tratamento de feridas. Rev Med 2010
- Posthauer ME, Dorner B, Collins N. Nutrition: A Critical Component of Wound Healing. Adv Skin Wound Care 2010
- Ferguson RP, O’Connor P, Crabtree B, Batchelor A, Mitchell J, Coppola D. Serum albumin and prealbumin as predictors of hospitalized elderly nursing home residents. J Am Geriatr Soc. 1993; 41:545-549.
- Watters CA, Tredget EE. Nutrition and wound healing. The Canadian Journal of CME 2002; 65– 70
- Brenner D.A., Buck M., Feitelberg S.P., Chojkier M. Tumor necrosis factor‐α inhibits albumin gene expression in a murine model of cachexia. J Clin Invest 1990;85: 248–55
- Moshage HJ, Janssen J, Franssen JH, Hafkenscheid JCM, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest 79 1987:1635– 41
- Ganong WF. Dynamics of blood and lymph flow. In: Review of Medical Physiology, 17th ed.Connecticut, Appleton and Lange, 1995;525–41
- Hoye RC, Bennett SH, Geelhoed GW, Gorschboth C. Fluid volume and albumin kinetics occurring with major surgery. J Am Med Assoc 1972;222:1255–61
- Chohan ND. Skillmasters: Wound care. LWW medical book collection Skillmasters Series. ed Lippincott Williams & Wilkins 2007
- Apelgren KN, Rombeau JL, Twomey PL, Miller RA. Comparison of nutritional indices and outcomes in critically ill patients. Crit Care Med (1982;(10):305–307
- Bradley JA, Cunningham KJ, Jackson VJ, Hamilton DNH, Ledingham IM. Serum protein levels in critically ill surgical patients. Intensive Care Med 1981;7:291–295
- Mouridsen HT. Turnover of human serum albumin before and after operations. Clin Sci.1967;33:345–54
- Chang Yu-Ling, Tsai Yun-Fang, Lin Pyng-Jing, Chen Min-Chi, Liu Chia-Yih. Prevalence and risk factors for postoperative delirium in a cardiovascular intensive care unit. Am J Crit Care. 2008; 17 (6):567–575.
- Ryan Aoife M, Hearty Aine, Prichard Ruth S, Cunningham Aileen, Rowley Suzanne P, Reynolds John V. Association of hypoalbuminemia on the first postoperative day and complications following esophagectomy. J Gastrointest Surg. 2007; 11(10):1355–1360. doi: 10.1007/s11605- 007-0223-y
- Ueda M, Endo I, Nakashima M, Minami Y, Takeda K, Matsuo K, Nagano Y, Tanaka K, Ichikawa Y, Togo S, Kunisaki C, Shimada H. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009; 33(1):104–110. doi: 10.1007/s00268-008-9807-2.
- Redelmeier DA. New thinking about postoperative hypoalbuminemia: a hypothesis of occult protein-losing enteropathy. Open Medicine 2009; 3(4): 215-e219.
- Abt E et al.Removal of beningn intraoral masses using the CO2 laser. J Am Dent Assoc.1987:729 – 31.
- Barak S, Kaplan I. The use of the CO2 laser in removing tumors of the oral cavity.. Laser Surg Med, New York. 1988: 97-98.
- Catone GA. Laser applications in oral and maxillofacial surgery.Saunders Company, Philadelphia 1997:307.
- Basu MK, Frane JW, Evans PH. Wound healing following partial glossectomy using the CO2 laser diathermy and scalpel: a histological study in rats. J Laryngol Otol. 1986:547.
- Debias DA, et al. Healing of incisions in the tougue: a comparison of results with milliwatt carbon dioxide laser tissue welding versus suture repair. Ann Otol Rhinol Laryngol. 1994: 964- 974.
- Clark CW. Comparative observations on effects of carbon dioxide laser induced peripheral nerve lesions in the rat. Surg Neurol. 1983:144.
- How ACSW, Ong CCP, Jacobsen A, Joseph VT. Carbon dioxide laser circumcisions for children. Pediatric Surgery International 2003; 19(1-2):11-3. DOI:10.1007/s00383-002-0894-0
- Trelles MA, Kaplan I, Rigau J, Buezo O. Periocular skin reshaping by CO2 laser coagulation. Aesthetic Plast Surg. 1996; 20(4):327-31.
- Krupa Shankar D., Chakravarthi M., Shilpakar R.. Carbon Dioxide Laser Guidelines. Journal of Cutaneous and Aesthetic Surgery. 2009; 2(2):72-80. doi:10.4103/0974-2077.58519.
- Raghuveer C., Murthy C., Siddalingappa K., Shivanand D.R. Multiple Trichoepitheliomas: Cosmetic Improvement with Dermabrasion. Journal of Cutaneous and Aesthetic Surgery. 2011; 4(1): 68-69. doi:10.4103/0974-2077.79203.