Information
Journal Policies
Impact of Extra-Nasal testing site on the Screening of Methicillin- sensitive Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus Colonization among HIV-Positive Individuals
Goyitom Gebremedhn1*, Haftom Niguse2, Muthupandian Saravanan3
2.School of Pharmacy, College of Health Sciences, Mekelle University, Mekelle, Ethiopia.
3.Department of Medical Microbiology and Immunology, Division of Biomedical Sciences, School of Medicine, College of Health Sciences, Mekelle University, Mekelle, Ethiopia.
Copyright : © 2019 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Background: Even though there are numerous studies regarding prevalence and risk factor of MSSA and MRSA colonization, local data related to the impact of additional testing site on the screening of colonized persons is limited. Bearing this in mind, we undertook this study in a hospital to determine the impact of additional anatomical site other than the anterior nares for the screening sensitivity among population group at risk of MSSA and MRSA colonization; HIV-infected persons attending the hospital for follow-ups.
Methods: This was a cross-sectional study in which impact of Extra-nasal testing body site on the sensitivity of detecting colonized persons was determined. A well structured data collection format was used to collect socio-demographic characteristics of HIV positive individuals. A total of 498 Nasal and throat swabs (two from each participant) were collected from 249 participants, transported and processed using standard bacteriological procedure.
Results: MSSA was isolated from 81 (32.5 %) patients, with MRSA colonization rate of 6 (2.4 %). The inclusion of throat swabs increased the sensitivity of nasal screening of MSSA and MRSA by 34.6% and 33.3% respectively.
Conclusion: Our study finding indicates that the sensitivity of MSSA/MRSA colonization is better enhanced when multiple-site screening is implemented in colonized persons.
Keywords: Methicillin-Sensitive Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, HIV, Colonization.
1. Introduction
Staphylococcus aureus, even though a commensal both on human skin and the mucosa, it is a frequent cause of serious infections with high morbidity, mortality, and health-care associated costs [1]. Since it was first discovered in Britain in the 1960s, Methicillin resistant Staphylococcus aureus (MRSA) infections have become increasingly problematic in both health care and community settings, leading to a greater morbidity, mortality, longer hospital stays, prolonged antibiotic administrations and increased treatment costs [2-5].
The anterior nares are the most frequent carriage site, which serves as reservoir for the spread of pathogens [3,6,7,8]. S.aureus/MRSA can also colonize the axilla, inguinal region, throat, oropharynx, wounds and gastrointestinal tract [7-10].
Though S.aureus/MRSA can infect all patients, HIV infected patients are more susceptible to MRSA due to their compromised immune system, frequent exposure to health care facilities, frequent oral antibiotic usage and other behavioral risk factors [11-13]. Compared with the general population, HIV patients are six to 18-folds more susceptible to MRSA and it is the main cause of bacteremia and endocarditis in these patients [11-17].
Single-site swabbing for the screening of Methicillin-sensitive Staphylococcus aureus/MRSA colonization has a low sensitivity despite being recommended in some guidelines [18]. Therefore, the aim of this study was to determine the impact of combination of body sites (anterior nares and the throat) for detection of MSSA/MRSA colonization in HIV positive individuals.
2. Materials And Methods
A cross-sectional study was conducted from September 2014 to February 2015 in Mekelle Hospital ART clinic, Mekelle, Northern Ethiopia. The hospital is serving for about 800,500 population and giving treatment and follow-up for 4035 HIV-positive people.
All HIV-positive individuals who attend the hospital ART clinic during the study period were asked to participate and written consent was obtained from all volunteer participants. This study was ethically approved by the Ethical Review Committee (Ref. No- ERC0453/2014), College of Health Science Mekelle University.
A well structured data collection format was used to collect socio-demographic characteristics of HIV positive individuals.
Trained sample collectors collected nasal and throat swabs using sterile cotton swabs pre-moistened with sterile normal saline using standard procedure [19-21]. A single sterile cotton swab was used by rotating 2-3 times inside the anterior nares to collect the nasal swab, and the throat swab was collected by another sterile cotton swab by swabbing the posterior pharynx and lateral walls of the pharynx (tonsillar area), without touching the buccal mucosa or tongue [22]. Stuart’s transport medium inoculated one with the nasal and a second from the throat swabs were used to transport the specimens to where the whole microbiological analyses were conducted within 3-4 hours of collection.
A selective medium Mannitol Salt Agar (MSA) (Oxiod, Hampshire, UK) were used to isolate S. aureus (MSSA and MRSA) and incubated at 370c aerobically for 24 hours. Typical colonies which are yellowish colonies from the MSA plate were sub-cultured onto Nutrient Agar (Oxiod, Hampshire, UK) for further biochemical characterization. Tube coagulase test was used to differentiate coagulase negatives from coagulase positive (S. aureus). MRSA isolates were identified using Cefoxitin disc by the Kirby-Bauer disk diffusion method [23].
Antimicrobial susceptibility testing was performed using the modified Kirby- Bauer disk diffusion method according to the clinical laboratory standard institute (CLSI) guidelines [23].
Demographic and laboratory data were entered into a computer and statistical analysis was done using SPSS version 20.0.
Sample collection, transportation and processing steps were performed following Standard operating procedures (SOPs). American Type Culture Collection (ATCC) reference strain of S. aureus ATCC 25923 were used to test the quality of of the culture media and antimicrobial disks.
3. Results
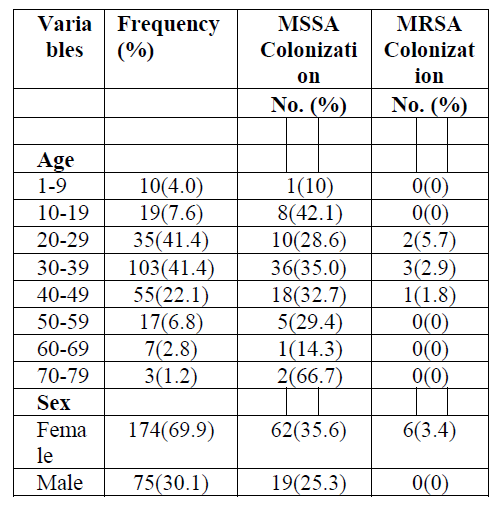
A total of 249 HIV positive individuals attending HIV care service were included in the study. Among the study participants, females account for 174 (69.9%), and 75 (31.1%) were males. The participants were in the age range of 5-72 years with a mean age of 35 years and the age group of 30-39 contained majority of the participants (103(41.4%)) (Table 1).
MSSA and MRSA colonization rate were 81(32.5%) and 6(2.4%). The age group 30-39 accounts the higher MSSA and MRSA colonization in which 36 of the total 81 MSSA colonization and 3 of the total MRSA colonization were found in. Whereas the least MSSA colonization were in the age groups of 1- 9 and 60-69, where only a single person from each group was found colonized. Both MSSA and MRSA colonization were higher in females, in which 62 of the total MSSA and all the 6 MRSA were found.
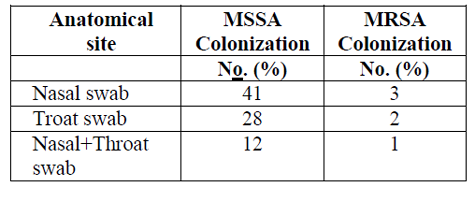
The detection of both MSSA and MRSA colonization was more pronounced when swabs from two different anatomical sites were used. From the total 81 MSSA colonized participants, the distribution by body site were, 41 (50.6%) in the nasal swabs, 28 (34.6%) in the throat swabs and 12 (14.8%) in both nasal and throat swabs. Were as among the 6 participants found colonized with MRSA, the distribution by nasal, throat and both sites were 3, 2 and 1 respectively (Table2).
The antimicrobial susceptibility testing for the MRSA isolates against the commonly used antimicrobials Ciprofloxacin (5µg), Trimethoprim-Sulfamethoxazole (1.25/23.75 μg), Erythromycin (15 μg), Clindamycin (2 μg), and Amikacin (30 μg) were performed. Among the 6 MRSA isolates, 1 (16.7%) were found resistant to Ciprofloxacin, 2 (33.3%) to Trimethoprim-Sulphamethaxazole, and 3 (50%) to Clindamycin and Erythromycin. unlike to other antimicrobials, all the isolates were sensitive to Amikacin.
4. Discussion
Patients colonized with MSSA or/and MRSA are the main reservoir in health facilities and about 35%-84% of these colonized patients are missed during screening tests [23, 24]. These undetected but colonized patients aggravate the risk of cross transmission of MSSA and MRSA hospital acquired infections [25, 26]. To tackle this low sensitivity of single-site testing for the screening of MSSA/MRSA colonization, various countries have different policies on the number of sites for the screening of MSSA/MRSA colonization.
The sensitivity of MSSA/MRSA colonization is not uniform on different patients’ anatomical sites like the anterior nares, troat, axilla, groin, skin and other testing sites, in which the throat and nares show higher colonization detection [27-29]. In this study, we assessed the impact of extra nasal anatomical site for the screening of MSSA/MRSA colonization in a population at high risk. Two separate swabs from the nares and throat were collected from each participant to determine the sensitivity of detection.
In this study the sensitivity of MSSA colonization increases when combination of two separate swabs, one from the nares and one from the throat is used. The increment was 28(34.6%) than when used only nasal swabs, which was also indicated by other studies [30-32].
Similar to the MSSA, the sensitivity of MRSA colonization increases by 33.3 % when the extra-nasal throat swab was added to screen the patients. This is also supported by other studies from different areas [27-29].
5. Conclusion
Our study shows the need for the extra-nasal anatomical testing site for the screening of MSSA/MRSA carriers which could be valuable for control of MSSA/MRSA cross transmission and hospital infections. This increase in the sensitivity of detection of colonized patients by the addition of extra-nasal testing sites is of immense value, particularly for susceptible population groups, such as HIV-infected persons, patients at hemodialysis units and other immune compromised groups.
6. Acknowledgments
We would like to appreciate to the Tigray Regional Health Bureau and Tigray Health and Research Laboratory for financial and resource support. We would also like to give a heartfelt gratitude to staff members of the ART clinic in Mekelle Hospital for their support during data collection, to the study participants, and the staffs of microbiology laboratory of the Ayder Referral Hospital.
7. Authors’ Contributions
GG was the principal investigator, conceived the study, designed the data collection, laboratory works, data analyzed and drafted the manuscript for publication. HN and MS have participated in data analysis and preparation of the manuscript.
References
- Schmidt, A., Bénard, S., Cyr, S.. Hospital cost of staphylococcal infection after cardiothoracic or orthopedic operations in France: a retrospective database analysis. Surg. Infect. 2015; 16(4):428–435. doi: 10.1089/sur.2014.045.
- Government of Western Australia Department of Health. Infection Prevention and Control of methicillin-resistant Staphylococcus aureus (MRSA) in Western Australian Healthcare Facilities. Western Australia: Healthcare Associated Infection Unit (HAIU), Communicable Disease Control Directorate, Department of Health; 2013.
- Wertheim HF, Melles DC, Vos MC et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005; 5(12):751– 762. doi: 10.1016/S1473-3099(05) 70295-4.
- Filice GA, Nyman JA, Lexau C, et al. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect Control Hosp Epidemiol 2010; 31(4):365–373. doi: 10.1086/651094.
- Edem EN, Onwuezobe IA, Ochang EA, Etok CA, Eyakndue EO. Antibiogram of nasal isolates of Staphylococci in anterior nares of human immunodeficiency virus patients in the University of Uyo Teaching Hospital (UUTH) Uyo, Akwa Ibom State, Nigeria. J Microbiol Microb Res 2013; 1:7–12.
- Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev 1963; 27:56–71.
- Sivaraman K, Venkataraman N, Cole AM. Staphylococcus aureus nasal carriage and its contributing factors. Future Microbiol 2009; 4(8):999–1008. doi: 10.2217/fmb.09.798.
- Otto M. MRSA virulence and spread. Cell Microbiol 2012; 14(10):1513–1521. doi: 10.1111/j.1462-5822.2012.01832.x
- Shahin R, Johnson IL, Jamieson F, Mcgeer A, Tolkin J, Ford-Jones EL (1999) Methicillin-resistant Staphylococcus aureus carriage in a child care following a case of disease. Arch Pediatr Adolesc Med 1999; 153(8):864–868
- Eveillard M, De Lassence A, Lancien E, Barnaud G, Ricard JD, Joly-Guillou ML. Evaluation of a strategy of screening multiple anatomical sites for methicillin-resistant Staphylococcus aureus at admission to a Teaching Hospital. Infect Control Hosp Epidemiol 2006; 27(2):181– 184
- Tumbarello M, de Gaetano Donati K, Tacconelli E et al. Risk factors and predictors of mortality of methicillin resistant Staphylococcus aureus (MRSA) bacteraemia in HIV-infected patients. J Antimicrob Chemother 2002; 50(3):375–382.
- Onorato M, Borucki MJ, Baillargeon G, et al. Risk factors for colonization or infection due to methicillin-resistant Staphylococcus aureus in HIV-positive patients: a retrospective case– control study. Infect Control Hosp Epidemiol 1999; 20(1):26–30.
- Villacian JS, Barkham T, Earnest A, Paton NI. Prevalence of and risk factors for nasal colonization with Staphylococcus aureus among human immunodeficiency virus-positive outpatients in Singapore. Infect Control Hosp Epidemiol 2004; 25(5):438–440.
- Burkey MD, Wilson LE, Moore RD, Lucas GM, Francis J, Gebo KA. The incidence of and risk factors for MRSA bacteremia in an HIV-infected cohort in the HAART era. HIV Med 2008; 9(10):858-862. doi: 10.1111/j.1468-1293.2008.00629.x.
- Franzetti F, Grassini A, Piazza M, et al. (2006) Nosocomial bacterial pneumonia in HIV-infected patients: risk factors for adverse outcome and implications for rational empiric antibiotic therapy. Infection 2006; 34(1):9– 16.doi:10.1007/s15010-006-5007-x.
- Furuno JP, Johnson JK, Schweizer ML, et al. Community-associated methicillin resistant Staphylococcus aureus bacteremia and endocarditis among HIV patients: a cohort study. BMC Infect Dis 2011; 11:1–7. doi: 10.1186/1471-2334-11-298.
- Popovich KJ, Weinstein RA, Aroutcheva A, Rice T, Hota B. Community-associated methicillin resistant Staphylococcus aureus and HIV: interesting epidemics. Clin Infect Dis 2010; 50(7):979-987. doi: 10.1086/651076 .
- Jernigan J, Kallen A. Methicillin-resistant Staphylococcus aureus (MRSA) infections, activity C: ELC prevention collaboratives. 2010. Available from: http:// www.cdc.gov/HAI/pdfs/toolkits/MRSA_ toolkit_ white_020910_ v2.pdf.
- D’Avila NE, Zhang L, Miller RG, D’Avila AC, Conceição AP, Boffo MS. High prevalence of nasopharyngeal colonization by Staphylococcus aureus among children with HIV-1 infection in extreme southern Brazil. J Trop Pediatr 2008; 54(6):410–412 doi: 10.1093/tropej/fmn051
- Dhuria N, Devi P, Devi B, Malhotra S. Prevalence and risk factors for methicillin-resistant Staphylococcus aureus colonization in anterior nares of HIV- positive individuals. Wudpecker J Med Sci 2013; 2(3):026–029
- Kumar S, Bandopadhyay M, Banerjee P, Laskar S. Nasal methicillin-resistant Staphylococcus aureus colonization in HIV-infected patients from Eastern India. Saudi J Health Sci 2013; 2(1):14–17
- CLSI. Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S23. Clinical and Laboratory Standards Institute, Wayne, 201
- Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368(9538):874–885. doi:10.1016/S0140-6736(06)68853-3
- Struelens MJ, Hawkey PM, French GL, Witte W, Tacconelli E. Laboratory tools and strategies for methicillin-resistant Staphylococcus aureus screening, surveillance and typing: state of the art and unmet needs. Clin Microbiol Infect 2009; 15(2):112–119.
- Fishbain HT, Lee JC, Nguyen HD, et al. Nosocomial transmission of methicillin-resistant Staphylococcus aureus: a blinded study to establish baseline acquisition rates. Infect Control Hosp Epidemiol 2003; 24(6):415–421.
- Lucet JC, Chevret S, Durand-Zaleski I, Chastang C, Regnier B. Prevalence and risk factors for carriage of methicillin-resistant Staphylococcus aureus at admission to the intensive care unit. Arch Intern Med 2003;163(2):181–188.
- El-Bouri K, El-Bouri W. Screening cultures for detection of methicillin-resistant Staphylococcus aureus in a population at high risk for MRSA colonization: identification of optimal combinations of anatomical sites. LJM 2013;8:22755.
- Jang H, Choi O, Kim G, et al. Active surveillance of the trachea or throat MRSA is more sensitive than nasal surveillance and a better predictor of MRSA infections among patients in intensive care. PLoS One 2014;9(6):e99192.
- Lauderdale TLY, Wang JT, Lee WS, et al. Carriage rates of methicillin-resistant Staphylococcus aureus (MRSA) depend on anatomic location, the number of sites cultured, culture methods, and the distribution of clonotypes. Eur J Clin Microbiol Infect Dis 2010; 29(12):1553–1559. doi: 10.1007/s10096-010-1042-8
- Chow A, Win MK, Wong CS, Leo YS. Universal methicillin-resistant Staphylococcus aureus (MRSA) screening: comparison of anatomic screening sites for patients with high and low prevalence of MRSA carriage. Infect Control Hosp Epidemiol 2012; 33(3):315–317. doi:1086/664042.
- Kyaw WM, Lee LK, Siong WC, Li Ping AC, Ang B, Leo YS. Prevalence of and risk factors for MRSA colonization in HIV positive outpatients in Singapore. AIDS Res Ther 2012; 9:1–doi:10.1186/1742-6405-9-33
- Mertz D, Frei R, Jaussi B, et al. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis 2007; 45(4):475–477. doi:10.1086/520016