Information
Journal Policies
Evaluation of the Efficacy of the Phyto-Drug Djovikas: Traditional Treatment against HIV Infection
Erick Ntambwe Kamangu1,2 *,Ben Ilunga Bulanda2, Berry Ikolango Bongenya2, Elvis Tshunza Kateba2,3, Medard Omakoy Okonda2, Victor Nyima Kasongo4, Michelline Abiba Kingombe3
2."HIV/AIDS Focus" Research Group, Kinshasa - Democratic Republic of Congo.
3.National Program for the Promotion of Traditional Medicine and Medicinal Plants (PNMT-PM), Ministry of Public Health, Kinshasa-Democratic Republic of Congo.
4.Centre Tradi-moderne Bonkoko, Kinshasa-Democratic Republic of Congo.
Copyright : © 2019 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: "Djovikas" is a phyto-drug based on local plants. It has been consumed for many years by a large number of people living with the Human Immunodeficiency Virus (PLHIV) in the city of Kinshasa and in other cities in the country.
Objective: The objective of this work was to evaluate the efficacy of the phyto-drug "Djovikas" 6 months after the beginning of the treatment of the patients.
Methods: Prospective cohort of 6 months on patients followed by traditional medicine. The participation in this work was voluntary. Ninety-seven (97) patients diagnosed with HIV-1 by serology were included in D0 at this work. All patients signed consentform for participation in the study. At the 6thmonth appointment, 48 patients returned for their clinical and biological evaluations. Clinical parameters were evaluated according to the WHO Clinical Stage classification for HIV infection. The biological parameters of the patients had been carried out in the same laboratories and under the same conditions.
Results: Out of 97 patients included in D0, 48 patients returned for their M6 appointment. This population was made up of 35 women and 13 men. The median age was of 41 years with extremes of 21 and 60 years. Forty-five patients (93.75%) were in Clinical Stage 2 according t o WHO at the 6thmonth, followed by 3 patients (6.25%) in Clinical Stage 3. A Nested DNA PCR, to confirm the patient's status, was positive in all patients. The median values of Viral Loads (VL) and CD4 lymphocyte count at month 6 were 1.56 log10RNA copies/ml (36.50 RNA copies/ml) and 456 cells/ml, respectively.
Conclusion: The phyto-drug "Djovikas" proved to be effective in the treatment of HIV infection. After 6 months of treatment, the patients' VLs fell sharply and their immunity was restored after taking the drug.
Keywords: HIV, Djovikas, Phyto-drug, Efficiency, Kinshasa.
Abbreviations : AHT: African Human Trypanosomiasis, AIDS: Acquired Immuno Deficiency Syndrome, ART: Anti Retro
Viral Treatment, ARV: Anti Retro Viral, ATC: Ambulatory Treatment Center, BMI: Body Mass Index, DNA: Deoxyribose Nucleic Acid, HIV: Human Immunodeficiency Virus, PCR: Polymerase Chain Reaction, PD: Phyto-Drug, PLHIV: People Living with the Human Immunodeficiency Virus, RNA: Ribose Nucleic Acid, UNIKIN: University of Kinshasa, VL: Viral Load, WHO: World Health Organization
1. Background
"Djovikas" is a phyto-drug (PD) based on local plants. It has been consumed for many years by a large number of People living with the Human Immunodeficiency Virus (PLHIV) in Kinshasa and in other neighboring cities [1].
In 2012, only 15% of people eligible for antiretroviral treatment (ART) currently had access to this treatment [1]. This situation had opened the door to finding alternative treatment for HIV infection by those patients who are the first to be concerned in cases of insufficiency and out-of-stock of ART.
Tradi-modern medicine is already widely used in many African countries to fight HIV infection [2,-4]. In Kinshasa, several works have demonstrated the effectiveness of plants and phyto-drugs against malaria [5], African Human Trypanosomiasis (AHT) [6] and other pathologies.
Nevertheless, there is no concrete data on the effectiveness of traditional medicine in the fight against HIV and AIDS in our environment. Hence the objective of this work was to evaluate the effectiveness of the phyto-drug "Djovikas" 6 months after the beginning of the treatment of the patients.
2. Methods
The present work was a prospective 6-month cohort study on patients followed by traditional medicine. The inclusion period was from January 11thto March 11th, 2016.
Ninety-seven (97) patients diagnosed with HIV Type 1 by serology were included in D0 in this work. They were recruited voluntarily at the Bonkoko Tradi-Modern Center in Kinshasa. The inclusion criteria for the subjects were: (i) to be diagnosed HIV-1 positive according to national guidelines [7], (ii) to be over 18 years of age on the date of inclusion, and (iii) to be eligible for antiretroviral treatment (ART). Patient demographic, clinical and paraclinical information were recorded on the pre-tested survey forms for the study. Participation in this work was voluntary. All patients signed informed consent for participation in the study.
At the 6thmonth (M6) appointment, only 48 patients returned for their clinical and biological evaluations at the treatment center.
Age, sex, height, weight, and Body Mass Index (BMI) were the Anthropometric monitoring parameters that were assessed at baseline (D0), 1st, 3rdand 6thmonth (M1, M3 and M6) by clinicians. This information was recorded on individual patient files.
Clinical parameters were evaluated according to the World Health Organization (WHO) Clinical Stage classification for HIV infection [7]. These parameters were taken by the clinicians and recorded on the patient's individual files.
The biological parameters of the patients had been carried out in the same laboratories and under the same conditions for D0, M1, M3 and M6. The results of the analyzes were recorded on the patient's individual files. The biological parameters of interest were: CD4 count, Viral Load (VL), Amylase, urea, creatinine, blood glucose, total cholesterol, LDL, HDL, triglyceride, transaminases SGPT and SGOT.
At inclusion (D0) and at the 6thmonth (M6), 2 blood samples were taken from each patient from the elbow crease vein: one 5ml sample in one tube with EDTA anticoagulant and the other one 5ml with Lithium Heparin. The EDTA tube was used for CD4 count and VL while the Lithium tube was used for biochemistry analyzes (amylase, urea, creatinine, blood glucose, total cholesterol, LDL, HDL, triglyceride, SGPT transaminases and SGOT). On D0, the Buffy coat taken from the EDTA tube was used to confirm the serological status of the patients using a Nested PCR previously described [8]. The VLs, as well as the confirmation PCRs, were carried out at the Molecular Biology Laboratory of the Faculty of Medicine of the University of Kinshasa (UNIKIN) using the specific techniques previously described [8,9]. While the biochemistry parameters were evaluated in the laboratory of Biochemistry of the Faculty of Pharmaceutical Sciences of UNIKIN.
3. Results
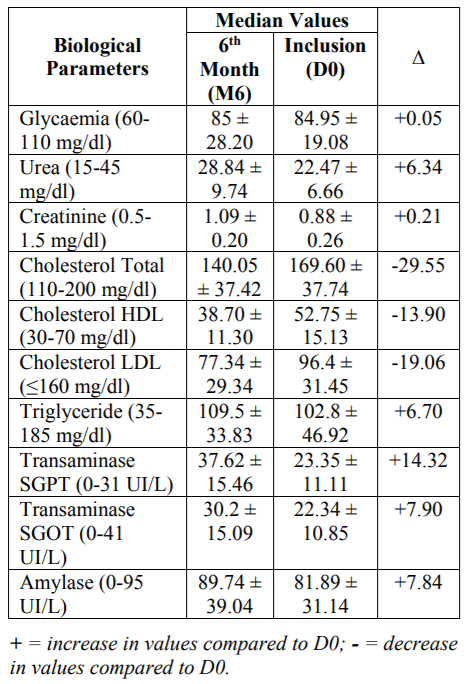
Out of 97 patients included in D0, 48 patients returned for their M6 appointment. This population was made up of 35 women and 13 men. The median age was 41 years with extremes of 21 and 60 years. Forty-five patients (93.75%) were in Clinical Stage 2 according to WHO 6 month after starting treatment, followed by 3 patients (6.25%) in Clinical Stage 3. Nested DNA PCR, to confirm the patient's status, was positive in all patients. The median values of VL and CD4 lymphocyte count at month 6 were 1.56 log10RNA copies/ml (36.50 RNA copies /ml) and 456 cells/ml, respectively. The results of the different biological analyzes carried out for M6 are presented in the following table 1.
4. Discussion
The objective of this work was to determine the efficacy of the phyto-drug "Djovikas" as traditional treatment against HIV infection used in Kinshasa.
Out of 97 patients included in D0, 48 patients returned for their 6thmonth appointment. This population was made up of 35 women and 13 men. The loss rate of the patients was 50.5% compared to the total number of patients at the beginning of treatment. The losses of patients were generally attributed to the loss of sight in the centers but also because of deaths in outpatient treatment centers (ATC) for the monitoring of People Living with HIV (PLHIV) in our environment [10]. In this work, beyond known reasons, perditions can also be caused by the society and its lack of adherence to traditional medicine [2].
After 6 months of treatment, 45 patients (93.75%) were in Clinical Stage 2 according to the WHO and 3 patients (6.25%) in Clinical Stage 3. No patients evolved into Clinical Stage 4. Compared with patient’s data at the inclusion [11], patients' health status did not deteriorate using traditional treatment with "Djovikas".
From a clinical point of view, this drug is well tolerated by patients and has not caused any failure.
The main goal of Anti Retro Viral (ART) therapy is to suppress viral replication or reduce plasma VL by making it undetectable, and restore patient immunity which results in increased T4 lymphocytes (CD4). After 6 of treatment with "Djovikas", the median values of VLs and CD4 Lymphocyte levels were respectively 1.56 log10RNA copies/ml (36.50 RNA copies/ml) and 456 cells/ml. At inclusion, the median VL and CD4 values were 4.10 log10RNA copies/ml and 220 cells/ml, respectively [11]. These results show a clear improvement in the state of health of patients taking the phyto-drug.
The results of the different biological analyzes carried out for M6 were presented in the table 1. All average values obtained are in the limits of the parameters (Blood glucose: 85 ± 28.20 mg/dl, Urea: 28.84 ± 9.74 mg/dl, Creatinine: 1.09 ± 0.20 mg/dl, Total cholesterol: 140.05 ± 37.42 mg/dl, HDL cholesterol: 38.70 ± 11.30 mg/dl, LDL cholesterol: 77.34 ± 29.34 mg/dl, Triglyceride: 109.5 ± 33.83 mg/dl, SGPT Transaminase: 37.62 ± 15.46 IU/L, SGOT Transaminase: 30.2 ± 15.09 IU/L, Amylase: 89.74 ± 39.04 IU/L). Compared to the mean values recorded in D0 [11], there was a clear, though not significant, increase in urea (Δ = 6.34 mg/dl), creatinine (Δ = 0.21 mg/dl), triglyceride (Δ = 6.7 mg/dl), SGPT (Δ = 14.32 IU/L), SGOT (Δ = 7.9 IU/L) and amylase (Δ = 7.84 IU/L); and a remarkable decrease in total cholesterol (Δ = -29.55 mg/dl), HDL (Δ = -13.9 mg/dl) and LDL (Δ = -19.06 mg/dl).
5. Conclusion
The phyto-drug "Djovikas" proved to be effective in the treatment of HIV infection in our environment. After 6month of treatment, the patients' VLs decrease sharply and their immunity was restored after taking the phyto-drug compared to the start of treatment.
6. Authors’ Contribution
ENK, BIB1, BIB2, ETK and MAK conceived and designed the study. ENK, BIB1, BIB2, ETK, MOO and VNK acquired the data. ENK, BIB1, BIB2, ETK and MOO analyzed and interpreted the data. ENK, BIB1, BIB2 and ETK drafted the manuscript. ENK, BIB1, BIB 2, ETK, MOO and MAK revised the manuscript. ENK, BIB1, BIB2, ETK and MOO approved the final version of the manuscript. All authors read and approved the final manuscript.
References
- Kamangu NE, Nyima KV, Kingombe AM, Nkutsi TW, Mesia KG. Suivi des patients VIH positifs traités par la Médecine Traditionnelle à Kinshasa : étude rétrospective des dossiers des patients suivis au Centre BONKOKO. Revue Congolaise des Sciences. 2013; 1 (2): 50-9.
- Makita-Ikouaya E, Milleliri JM, Rudant JP. Place de la médecine traditionnelle dans le système de soins des villes d’Afrique subsaharienne : le cas de Libreville au Gabon. Cahiers Santé vol. 20, n° 4, octobre-novembre-décembre 2010: 179-88.
- Organisation Mondiale de la Santé. Médecine traditionnelle : besoins croissants et potentiels, perspectives politiques de l’OMS sur les médicaments. Genève: OMS, 2002.
- World Health Organization (WHO). Stratégie de l’OMS pour la Médecine Traditionnelle pour 2014-2023, Ouagadougou, 2010. http://www. who.int/
- Memvanga PB, Tona GL, Mesia GK, Lusakibanza MM, Cimanga RK. Antimalarial activity of medical plants from the Democratic Republic of Congo: A review. J Ethnopharmacol. 2015; 169: 76-98 https://doi: 10.1016/j/jep.2015.03.075
- Mvumbi LG, Nseka KN. Effets Antiplasmodium et Antitrypanosome de Manniphyton fulvum et Quassia africana. Annales Africaines de Médecine. 2004; 1: 73-79
- Programme National de Lutte contre le VIH/SIDA et les Infections Sexuellement Transmissibles (PNLS), Ministère de la Santé Publique, République Démocratique du Congo. Guide National de traitement de l’infection à VIH par les ARV chez l’adolescent et l’adulte,Révision 2016.
- Kamangu NE, Adawaye C, Boreux R, Kalala LR, Mvumbi LG, De Mol P, Vaira D, Hayette MP. Implementation of an In-House Quantitative Real-Time PCR for Determination of HIV Viral Load in Kinshasa. Open Access Library Journal. 2014; 1: e855. http://dx.doi.org/10.4236/oalib.1100855
- Kamangu NE, Mayemba C, Mbikayi S, Ndarabu A, Kalala LR, Mvumbi LG, Vaira D. Implementation of a Classic Nested PCR DNA for HIV Diagnosis in Kinshasa. International Journal of Collaborative Research on Internal Medicine and Public Health. 2014; 6 (6): 145-51.
- Kamangu NE, Kalala LR, Mvumbi LG, Vaira D, Hayette MP. Involvement of the Genetic Diversity of HIV-1 in the Virological Treatment Failure of First Line Antiretroviral in Kinshasa. World Journal of AIDS. 2017, 7, 23-33. https://doi.org/10.4236/wja.2017.71003
- Bulanda IB, Kateba TE, Bongenia IB, Kasongo NV, Kingombe AM, Kamangu NE. Sociodemographic and Anthropometric Profile of Positive HIV Patients in Early Traditional Treatment: Case of the Bonkoko Center. Open Access Library Journal. 2018; 5: e4555. https:// doi.org/10.4236/oalib.1104555