Information
Journal Policies
ARC Journal of AIDS
Volume-3 Issue-2, 2018, Page No: 1-2
Combined Caspofungin and Trimethoprim/ Sulfamethoxazole Therapy for Severe Pneumocystis Jirovecii Pneumoni
Mei-Chen Chen1, Chun-Chieh Yang2, Wen-Liang Yu2,3*
1.Department of Nursing, Chi Mei Medical Center, Tainan, Taiwan.
2.Department of Intensive Care Medicine, Chi Mei Medical Center, Tainan, Taiwan.
3.Department of Medicine, Taipei Medical University, Taipei, Taiwan.
2.Department of Intensive Care Medicine, Chi Mei Medical Center, Tainan, Taiwan.
3.Department of Medicine, Taipei Medical University, Taipei, Taiwan.
Citation : Mei-Chen Chen, Chun-Chieh Yang, Wen-Liang Yu, "Combined Caspofungin and Trimethoprim/ Sulfamethoxazole Therapy for Severe Pneumocystis Jirovecii Pneumoni" ARC Journal of AIDS. 2018; 3(2) : 1-2.
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Dear the Editor
Pneumocystis pneumonia (PCP) caused by Pneumocystis jirovecii (PJ) is a major cause of mortality and morbidity in immunocompromised patients, especially in HIV-positive persons. Sulfamethoxazole/ trimethoprim (SMZ/TMP) is first choice for drug of standard therapy or prophylaxis for PCP [1-3]. Caspofunginin combination with TMP/SMZ as salvage therapy for severe PCP was ever reported [1-4].
A 33 years old HIV-infected man was admitted to the hospital due to bilateral pneumonia with septic shock. Endotracheal intubation was performed with mechanical ventilation for acute respiratory failure on July 20, 2018.
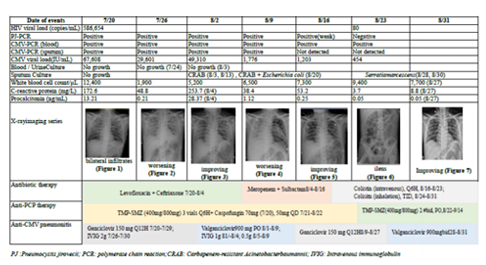
Laboratory data revealed a total lymphocyte count of 600/μL, with a CD4+ count of 20 % and CD8+, 57.9 % (CD4+/CD8+ ratio, 0.05). Arterial blood gas analysis showed a PH of 7.442; PCO2, 24.7 mmHg; PO2,149.0 mmHg; HCO3, 17.0 mmol/L; Base Excess, -4.4 mmol/ L; and P/F ratio, 149.0 mmHg under FiO2,100 %. Virology survey showed an HIV viral load of 586,654 copies/ml; cytomegalovirus (CMV) viral load,67, 608 IU/mL; and positive results of polymerase chain reaction (PCR)assay for CMV in blood and sputum samples. The result of DNA PCR for PJ was positive in transbronchial aspirates, revealing wild types of Thr55Ala and Pro57Ser by PJ DNA sequencing analysis (Table 1). Sputum MTB-PCR (GeneXpert assay) was negative. The blood and sputum cultures did not yield any bacterial growth. A chest X-ray (CXR) film showed ill-defined infiltration and haziness over both lung fields (Figure1, Table 1).
The patient received therapy with ceftriaxone and levofloxacin for suspected bacterial infection.SMZ/ TMP, methylprednisolone and caspofungin were initiated for PCP. Ganciclovir was added for CMVDNAemia with probable pneumonitis. However, pneumonia in worsening progression (Figure 2) accompanied with leukopenia (1900/μL) was noted (Table 1). Then IVIG was prescribed on July 26. PJP DNA PCR remained positive on August 2, 2018, but CXR film showed substantial improvement (Figure 3, Table 1). Intravenous ganciclovir was de-escalated to oral valganciclovir. However, a new onset of fever with an elevated procalciton in (PCT) level up to 28.37 ng/ mL (normal, < 0.05) occurred and carbapenem-resistant Acineto bacterbaumannii (CRAB) was isolated from sputum culture obtained on August 3.
Thenantibiotics with ceftriaxone and levofloxacin were shifted to meropenemand sulbactamin combination and subsequent colistin therapy alone. Combined therapy with SMZ/TMP and caspofungin was maintained or PCP. Nonetheless, worseningdyspnea was noted and the CXR film showed left pneumothorax, atelectasis of left lung and thus chest tube was inserted on August 9 (Figure 4, Table 1). Leukopenia got improvement after IVIG infusion therapy. Tracheostomy was performed on August 13. A followed-up CXR film showed improvement (Figure 5, Table1), and thereby training for weaning respiratory ventilator was started since August 15.
However, distended abdomen and vomiting were noted. Gastroparesis with ileus was considered (Figure 6, Table 1). Peripheral parenteral nutrition using SmofKabiven with glutamine was given. The CXR showed improving pneumothorax, and thus chest tube was removed on August 24. As negative PJ-PCR and a CMV viral load lowering to 454 IU/mL, caspofungin was discontinued and SMZ/TMP was shifted to oral-form SMZ/TMP. Thereafter, intravenous ganciclovir was shifted to oral valgancyclovir. Patient's respiration, CXR patterns and inflammatory parameters were better. He was transferred to the ward on August 29. After medical therapy, his lung condition was well controlled (Figure 7, Table 1) and he was then discharged on September 14, 2018. The patient has undergone regular follow up uneventfully for 3 months.
Lobo et al. demonstrated that the efficacy of caspofungin combined with the TMP/SMZ was significantly higher than that of each drug alone [2]. This combination may inhibit the entire life cycle of Pneumocystis jirovecii [4], and may achieve better outcomes if it is rapidly administered to patients as a first-line therapy, particularly in those requiring invasive mechanical ventilation [2,4]. In addition, the worsening hit of the course during August 4 to August 9 seemed due to nosocomial CRAB pneumonia and early de-escalation of intravenous ganciclovir to oral valganciclovir. These results imply that CMV might play a significant role of co-infection with PCP, further compromising the immunity of the patient and contributing to complexity and severe severity of the lung infections.
References
- Tu GW, Ju MJ, Xu M, Rong RM, He YZ, Xue ZG, Zhu TY, Luo Z. Combination of caspofungin and low-dose trimethoprim/ sulfamethoxazole for the treatment of severe Pneumocystis jirovecii pneumonia in renal transplant recipients. Nephrology (Carlton) 2013;18: 736–742.doi: 10.1111/nep.12133.
- Lobo ML, Esteves F, de Sousa B, Cardoso F, Cushion MT, Antunes F, Matos O. Therapeutic potential of caspofungin combined with trimethoprim-sulfamethoxazole for pneumocystis pneumonia: A pilot study in mice. PlosOne 2013;8: e70619. doi: 10.1371/ journal. pone.0070619.
- Huang YS, Yang JJ, Lee NY, Chen GJ, Ko WC, Sun HY, Hung CC. Treatment of Pneumocystis jirovecii pneumonia in HIV-infected patients: a review. Expert Rev Anti Infect Ther 2017; 15:873-892. doi: 10.1080/14787210. 2017.136 4 991.
- Zhang G, Chen M, Zhang S, Zhou H, Ji X, Cai J, Lou T, Cui W, Zhang N. Efficacy of caspofungin combined with trimethoprim/sulfamethoxazole as first-line therapy to treat non-HIV patients with severe pneumocystis pneumonia. ExpTher Med 2018; 15: 1594-1601. doi: 10.3892/etm. 2017.5516