Information
Journal Policies
Antiretroviral Therapy for Asymptomatic Adults and Adolescents with HIV-1 Infection and CD4+ T-Cell Counts ≥500 Cells/μL: A Systematic Review and Meta-Analysis
Andrew Anglemyer1,George Rutherford2,Hacsi Horvath3,Marco Vitoria Margaret Doherty4
2.Global Health Sciences, University of California, San Francisco, San Francisco, California, USA.
3.The Department of Hiv/Aids, World Health Organization, Geneva, Switzerland.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: World Health Organization (WHO) antiretroviral therapy (ART) guidelines are regularly updated with the most current evidence on when to initiate ART.
Methods: We performed a systematic review and meta-analysis and identified published literature and conference abstracts for randomised controlled trials (RCT) and cohort studies that compared HIV-infected patients starting ART at ≥ 500 CD4 cells/µL with those with < 500 CD4 cells/µL.
Results: We identified 24 articles. Studies found a decreased hazard of HIV disease progression with initiation at >500 CD4 cells/µL (2 RCTs: relative risk [RR]=0.38, 95% CI 0.20-0.74; 1 cohort: hazard ratio [HR]=0.20, 95% CI 0.10-0.42). One RCT found a reduced risk of HIV transmission (RR=0.11, 95% CI 0.06-0.19), although this was not supported in two cohorts (RR=1.17, 95% CI 0.46-2.98). There was no increased risk of most Grade 3/4 adverse events identified in RCTs, but there was evidence of increased risk of laboratory adverse events for earlier initiation (RR=1.43; 95% CI 1.13-1.81) from one cohort study.
Conclusion: We found moderate quality evidence that the ART initiation at CD4 ≥500 cells/µL leads to reduced risk of disease progression. The risk of adverse events for early initiators is not yet well understood and needs further investigation.
HIV, antiretroviral therapy, practice guidelines, World Health Organization,AIDS
1. Introduction
As a result of antiretroviral therapy’s (ART) clinical benefit in individuals infected with HIV-1 and its impact on the risk of HIV transmission, several HIV treatment guidelines committees have recommended initiating ART at earlier stages of HIV infection and at higher CD4 cell counts [1,2]. As early as 2014, the International Antiviral Society – USA [1] and the United States Department of Health and Human Services [3]recommend starting ART as close to diagnosis as possible without regard to clinical symptoms or degree of immune dysfunction. In contrast the World Health Organization’s (WHO) guidelines did not recommend starting all patients on HIV regardless of clinical signs and symptoms or CD4 cell level prior to September 2015 and restricted starting therapy to patients with fewer than 500 CD4 cells/µL or those with concurrent tuberculosis, advanced HIV disease (stage 3 or 4), pregnancy, age < 5 years, concurrent hepatitis B virus infection with severe liver disease or an HIV-infected person in a serodiscordant partnership [5]. The European AIDS Clinical Society and British HIV Association’s guidelines did not broaden to recommend treating all patients until 2015 [6].
Previously, the available literature was reviewed to assess the impact of early versus delayed treatment and found evidence that early ART initiation (baseline CD4 count between 350 and 500 cells/µl) was associated with a reduction in the risk of HIV progression or death, a reduction in risk of a non-AIDS defining illness and increased likelihood of immunologic recovery , but an increased risk of grade 3 or 4 laboratory abnormalities [7]. This current review updates this earlier review and re-evaluates the clinical and public health impact of earlier initiation of ART in HIV-1-infected patients using a higher threshold for treatment initiation (CD4 ≥ 500 cells/µl). These results were used to inform the 2016 WHO guidelines for use of antiretroviral drugs for the treatment and prevention of HIV infection.
2. Materials And Methods
We used Cochrane Collaboration methods to conduct a comprehensive and exhaustive search strategy [8]. We searched PubMed, CENTRAL, SCOPUS (including EMBASE 1996-present), Web of Science, and WHO’s Global Index Medi cus using Medical Subject Heading (MeSH) terms and a range of relevant keywords. The search period ranged from 1 January 1996 to 1 October 2016. The search strategy was iterative, in that references of included studies were searc hed for additional references. All languages were included. Additionally, we searched for potentially relevant abstracts from key scientific conferences (the Conference on Retroviruses and Opportunistic Infections, the International AIDS Conference, and International AIDS Soci ety Conference on HIV Pathogenesis, Treatment and Prevention) within the search period.
2.2.1. Inclusion Criteria
We included randomised controlled trials (RCT) with randomisation at either the individual or cluster level, non-randomised trials with alloca-tion at either the individual or cluster level and prospective cohort studies. Studies estima ting impact on clinical outcomes needed to have compared patients with HIV-infection and no other clinical indications for early ART plus CD4counts ≥500 cells/μL at the time of initia tion with patients whose CD4 cell counts were < 500 cells/μL at the time of initiation.
2.2.2. Exclusion criteria
We excluded single arm pre-post studies without distinct controls, case-control studies, cross-sectional studies and case series.
We imported search results into bibliographic citation management software (EndNote X4, Thomson Reuters, New York, New York USA) and excluded duplicate references. Reviewing only article titles, one author (HH) excluded all references that were clearly irrelevant. Two authors (GWR and AA), working independen-tly, reviewed titles, abstracts and descriptor terms of the remaining citations to identify potentially eligible reports. We obtained full text articles for all references identified as potent ially meeting inclusion criteria. GWR and AA reviewed these full text articles and applied inclusion criteria to establish each study's eligib-ility or ineligibility. Where information was incomplete, we contacted authors for additional data. After identifying trials for inclusion, GWR and AA extracted data from each study indepen-dently and entered these data into standardised data extraction forms and then compared extract ed data. There were no disagreements.
We used the Cochrane Collaboration tool for assessing risk of bias in the included RCTs. The Cochrane tool assesses risk of bias in individual studies in six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential biases. We used the Newcastle-Ottawa Scale to assess quality and risk of bias in the non-RCTs and observational studies [9]. This scale judges three general areas: selection of study groups, comparability of groups, and in the case of cohort studies ascertainment of outcomes.
We used published relative risks (RR) if provided in study reports and, when necessary, calculated RRs for dichotomous outcomes and the 95% confidence interval (CI). We pooled data across studies and estimated summary effect sizes using Review Manager 5.2 (Cochrane Collaboration, London, UK). Due to anticipated heterogeneity between study designs and populations, we modeled meta-analyses using a DerSimonian-Laird random-effects model. We present estimates of heterogeneity as I2; which is the percentage of variability in effect estimates due to heterogeneity rather than to chance.
The observational literature for two major outcomes, mortality and mortality or clinical progression, included a number of studies that reported data from the same cohorts. To minimise the problem of duplication of patients, we conducted a sensitivity analysis of studies with no or minimal overlap between cohorts.
We assessed the quality of evidence from the literature for each outcome using Grades of Recommendation Assessment, Development and Evaluation (GRADE) (Supplemental Table 1) . GRADE ranks the quality of evidence on four levels: high, moderate, low and very low. Data from randomised trials are considered.
3. Results
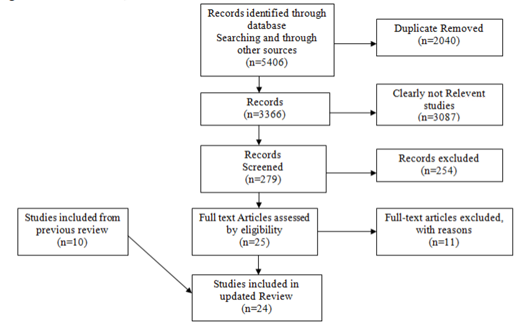
We identified 3366 unique reports and excluded 3087 (91•7%) based on titles and abstracts. Of the remaining 279 reports, 24 (8•6%) studies met inclusion criteria, 10 from our previous review and 14 new ones. Six reports were from three RCTs [11–16]; these included TEMPRANO , START and ANRS 12249 TasP. In two of these trials, START and ANRS 12249 TasP, patients randomised to not receive ART at CD4 counts >500 cells/µL did not initiate ART until their CD4 counts had fallen below 350 cells/µL, consistent with practice at the time the trials began. In TEMPRANO, the CD4 indication for to be of high quality but can be graded down for risk of bias, indirectness, inconsistency, imprecision or publication bias. Observational study data are considered to be of low quality, but can be graded up for large effect, if plausible confounding would increase confidence in an estimated effect or if there is a dose-response gradient.
beginning ART in the deferred treatment changed over time as WHO guidelines evolved from < 200 cells/µL to < 350 cells/µL to < 500 cells/µL in the absence of other clinical criteria [13]. Similarly participants in the early ART arm received ART when CD4 counts were >250 cells/µL, >350 cells/µL and >500 cells/µL as WHO guidelines changed ; unpublished analyses were made available to us for patients with>500 CD4 cells/µL at baseline. We also identified 18 observational studies (Figure 1)[17–34]. Studies were conducted in 40 countries across a range of high- and low-resource settings in Asia, the Americas, Europe and sub-Saharan Africa (Supplemental Table 2).
In two RCTs investigators reported no difference in the risk of mortality (pooled RR=0.73; 95% CI 0.39-1.36) . Seven observational studies comprising 55 cohorts also reported on mortality . Four studies found decreased risk of death in persons initiating ART at CD4 cell counts ≥500 cells/µL [35]. In reporting the seven studies, there was substantial overlap among the 55 cohorts, with data from 34 cohorts reported in ≥1 study. To obtain an appropriate pooled estimate we pooled data from one cohort that had not had its results reported from large synthetic cohorts with those of two other large studies that had the least overlap. These two studies from North America and Europe included 22and 23 cohorts respecti vely and comprised 20 972 participants. In pooled data, overlap was minimal, with only 154 (0.6%) participants reported in both analyses. There was no difference in the risk of mortality (RR 0.68, 95% CI 0.39-1.21) . The pooled point estimate across all seven observational studies was similar (RR 0.74, 95% CI 0.52-1.07).
Evidence quality for mortality from RCTs was very low; quality was downgraded for very serious imprecision and serious inconsistency due to conflicting results. Evidence quality in the observational literature was also very low, with downgrading for indirectness. Across all seven observational studies, evidence quality was also downgraded for risk of bias.
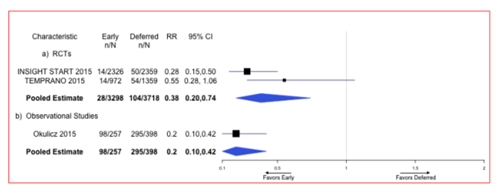
Two RCTs found lower risk of progressing to AIDS among those initiating ART at ≥500 CD4 cells/µL compared to those deferring until their CD4 counts were < 500 cells/µL . The pooled RR of developing AIDS from the RCT literature was 0•38 (95% CI 0•20-0•74) (Figure 2). Similarly, one observational study, which compared patients initiating ART at ≥500 CD4 cells/µL to those deferring until their CD4 counts were < 500 cells/µL, found a lower hazard of developing an AIDS-defining opportunistic infection among persons initiating ART early compared to those deferring treatment (HR=0•20; 95% CI 0•10-0•42) (Figure 2)[19].The quality of the RCT literature was moderate and downgraded as a result of imprecision due to few events. The quality of the observational literature was very low due to a lack of adjustment in the estimates.
One RCT estimated the effect of early (≥500 CD4 cells/µL) compared to deferred ART initiation (< 500 cells/µL) on the combined outcome of risk of death or severe HIV disease or incident malignancy . Patients who initiated treatment earlier had a lower hazard of death, severe HIV disease or malignancy than patients who deferred treatment (HR=0•59; 95% CI 0•33-1•06). Another RCT estimated the effect of early compared to deferred ART initiation on the combined outcome of risk of death or serious AIDS or serious non-AIDS event [15]. Patients who initiated treatment early had a reduced hazard of death, severe AIDS or serious non-AIDS event than those who deferred treatment (HR=0•43; 95% CI 0•30-0•62). The quality of the RCT literature for these outcomes was moderate and downgraded for imprecision.
Six observational studies estimated the effect of early versus deferred ART initiation on mortality or disease progression, and four found a decreased risk of progression to AIDS or mortality among early initiators; one study found a significant reduction .Early initiation of ART was not found to be associated with reduced risk of death or progression to AIDS in a pooled analysis of two studies with unique patients (RR=0•63; 95% CI 0•16–2•49) . However, data from one observational study (ICONA) [29] were fully contained in CASCADE 2011, and five of the 12 cohorts in a large HIV cohort collaboration (HIV-CAUSAL) were also contained in CASCADE 2011 [33]. An additional meta-analysis including the large HIV cohort collaboration in the pooled analysis and ignoring the non-independence of the data did not substantively change the results (RR=0•84; 95% CI 0•58-1•21). Among the observational studies, two of these five studies reported adjusted estimates Additionally, four of the five studies were consistent in their finding of treatment effect. The quality of the observational literature for this outcome is very low due to its observational status, inconsistency and risk of bias resulting from a lack of confounder adjustment.
One RCT found a decreased risk of transmission of HIV to sexual partners of patients treated early versus those who delayed treatment (RR=0•11; 95% CI 0•06-0•19) . Another RCT found no significant difference in risk between patients treated regardless of CD4 cells/µL and those who were treated according to the South African guidelines (aRR=0•95; 95% CI 0•79-1•14) [16]. Two observational studies also evaluated the risk of transmission; neither found a difference between patients treated early and those who received a delayed treatment (pooled RR=1•17; 0•46-2•98) . The quality of the RCT literature was very low owing to very serious imprecision because of the very small number of events and indirectness. The quality of the observational literature was also very low largely due to the lack of confounder adjustment in estimates and very small number of events.
One RCT reported specific severe adverse events in early and deferred treatment groups , while another RCT reported unspecified, symptomatic grade 4 events. Specifically, there was no noted increase in grade 3/4 laboratory abnormalities (HR=0•58; 95% 0•30-1•11) or hepatic (RR=0•62; 95% CI 0•20-1•80), renal (RR=0•09; 95% CI 0•01-1•63) or cardiovascular severe adverse events (RR=0•50; 95% CI 0•05-5•45), although there was a increased risk of adverse neurological events associated with early initiation (RR=4•96; 95% CI 1•09-22•53) [13]. Another RCT found no risk in symptomatic grade 4 severe adverse events between treatment arms (RR=1•01; 95% CI 0•73-1•40). The quality of the RCT literature for most outcomes, except grade 3/4 laboratory abnormalities and symptomatic grade 4 adverse events, was low due to low numbers of events and imprecise estimates. On the other hand, the quality of the RCT literature for the symptomatic grade 4 adverse event outcomes was high and moderate for grade 3/4 laboratory abnormalities.
One observational study compared reported severe adverse events between early and deferred treatment cohorts [17]. Investigators found an increased risk of any severe laboratory adverse event among those who were treated early when compared to those who initiated treatment with CD4 counts < 350 cells/µL (RR=1•43; 95% CI 1•13-1•81). Additionally, they found an increased risk of severe hepatic adverse events in those patients who initiated treatment early (RR=1•45; 95% CI 1•03-2•04). However, no differences were noted between treatment arms for renal (RR=0•90; 95% CI 0•40-2•01), haematologic (RR=1•40; 95% CI 0•87-2•26) or other severe adverse events (RR=1•40; 95% CI 0•94-2•08). The quality of the observational studies literature for these outcomes was low with no observed study limitations aside from the studies’ observational design.
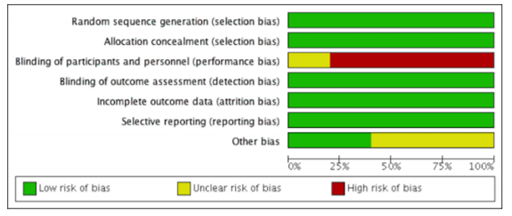
All six RCT reports adequately discussed how the randomisation sequence was generated, and all allocation was adequately concealed prior to assignment. No studies suffered from attrition bias resulting from incomplete outcome data reporting (e.g., follow-up in all studies was adequate), and no studies suffered from reporting bias resulting from selective outcome reporting. However, the trials may have potentially been biased because of a lack of blinding of assigned treatment (treatment was determined by pre-determined clinical characteristics and was open label) (Figure 3) [11–16].
No observational study suffered from obvious selection bias; all observational studies had study populations that were either truly or somewhat representative of average, HIV-infected persons, and all participants were drawn from the same community. Additionally, all treatment data were ascertained through health care records, and outcomes of interest were not present at the start of the study. Comparability between intervention and control arms was not strong as several studies did not adjust for confounding factors such as age or sex. Outcomes were adequately assessed in all studies either through independent blind assessment or record linkage. Follow-up was long enough for outcomes to occur in all studies, although six observational studies did not report follow-up rates of participants [17–20,22,31].
4. Conclusion
We found moderate quality evidence that early ART initiation in asymptomatic HIV-infected patients with baseline CD4 counts of ≥500 cells/µL is associated with a lower risk of disease progression. This was also found in the observational literature albeit with very low quality evidence. We found mixed evidence from two RCTs that early intervention was associated with lower risk of mortality or disease progression. Additionally, we found evidence of a reduced risk of HIV transmission, but this was of very low quality owing to very few events. Current WHO ART guidelines rate the quality of evidence for this outcome as high; those recommendations were, however, made based on the literature for serodiscordant couples in which the infected partners had ≥350 CD4 cells/µL [36]. The literature is substantially less robust for infected partners with ≥500 CD4 cell/µL, although the estimates of efficacy are quite similar. Finally we found high quality evidence from an RCT that early initiation of ART was not associated with an increased risk of symptomatic grade 4 adverse events. There is, however, low quality evidence from an observational study of an increased risk of hepatic adverse events; the clinical significance of this, especially in light of different findings in the RCT, is unclear.
The present review should be considered with its multiple limitations. As with all systematic reviews, the results are only as good as our identified literature-our ability to identify relevant studies. To reduce the possibility of missing key studies, we searched five targeted scientific databases and reviewed the bibliographies of included studies as well as abstracts from recent conferences. It should be noted, however, that the bulk of our identified literature comes from Europe, North America and Australia with only a few studies contributing data from Africa, Asia and Latin America, which may limit the generalisability of our results to the areas of the world with the greatest burden of disease. While publication bias is an ever-present risk in systematic reviews, the present review included large synthesized cohorts, (eg, NA-ACCORD, EUROSIDA, ICONA, CASCADE, HIV-CAUSAL), which may have reduced the likelihood of publication bias. Unfortunately, we identified too few studies to objectively test for publication bias.
Additionally, we calculated estimates of efficacy from RCTs and effectiveness from cohort studies. Three identified RCT reports used data from the same study population (TEMPRANO), and the incidence of some of the major clinical outcomes was particularly low. As a result, the precision of the estimates of mortality from RCTs, for example, was low; using GRADE criteria, the overall quality of the RCT literature for mortality was downgraded because of this. Additionally, we did not include data from HPTN 052, a seminal study that compared risk of transmission in discordant couples as well as clinical outcomes [38]. This is because the study population was restricted to HIV-infected index patients with between 350 and 550 CD4 cells/µL and compared early treatment in patients with 350-550 CD4 cells/µL with those with < 350 cells/µL. There has been no published analysis of patients who initiated with 500-550 cells, which would have been required for this systematic review.
Due to the lack of RCT data, and to be comprehensive in our data collection efforts, we also examined cohort studies. GRADE criteria dictate that cohort studies should provide a lower quality of evidence, and this is usually because of residual confounding. Our overall effectiveness estimates from the included cohort studies were likely biased as a result of a lack of independence between study populations, as we noted in our previous report . Namely, all seven studies reporting mortality outcomes contained data from some of the same cohorts . We attempted to minimise the lack of independence between studies by conducting sensitivity analyses, removing studies with the most overlap in populations. For example, for mortality we examined three studies--one from Italy , one large synthesized cohort from North America and one large synthesized cohort mostly from Europe , which had minimal overlap with each other. The point estimate from this synthesis (RR=0•68) was approximately nine percent lower than the point estimate from the pooled estimate from the full sample (RR=0•74), suggesting that the lack of independence between study populations did not greatly affect the point estimates. Expectedly, however, the variability decreased considerably with the much larger sample size. Additionally, cohorts can suffer from a number of biases, including confounding and lead-time bias. As a result of evaluating a subgroup of patients whose CD4 at ART initiation was greater than 500 cells/µL, which until recently was not routinely studied, much of our analyses were performed post-hoc using unadjusted estimates. Lastly, though the GRADE system for rating the quality of the literature has its limitations and some have reservations about its use at WHO for guideline development it is the current gold standard and has been adopted by WHO for its guideline development process [39].
In conclusion we found evidence from both RCTs and observational studies to support the WHO recommendation of initiating ART in everyone with HIV at diagnosis regardless of CD4 count or clinical stage. This strategy greatly simplifies ART prescribing and with sufficiently high coverage will impact HIV incidence as well. Additional data from studies still in the field, such as PopART[40]and SEARCH [41], as well as full publications from ANRS 12249 TasP, will provide additional evidence needed to answer the question of when to start ART definitively, both for individuals and at the population level.
Acknowledgements
We thank Dr Xavier Anglaret of the Université Victor Segalen Bordeaux 2 for sharing the additional analyses of TEMPRANO.
References
- Guenthard H, Aberg J, Eron J, et al. Antiretroviral treatment of adult HIV infection 2014 Recommendations of the International Antiviral Society-USA panel. JAMA 2014; 312:410–25.
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Second edition. Geneva: World Health Organization, 2016.
- Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. 2014. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines /adultandadolesc entgl.pdf. Accessed April 15, 2015.
- World Health Organization. Guidance on couples HIV testing and counselling including antiretroviral therapy for treatment and prevention in serodiscordant couples. Recommendations for a public health approach. Geneva: World Health Organization, 2012.
- Anglemyer A, Horvath T, Rutherford G. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA 2013; 310:1619–20.
- Williams I, Churchill D, Anderson J, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 HIV Med. 2012; S2:1-85.
- Anglemyer A, Rutherford GW, Easterbrook PJ, et al. Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS 2014; 28:S105– S1054.
- Higgins Julian PT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 ed. Oxford: Cochrane Collaboration; 2011.
- Wells G, Shea B, O'Connell, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non- randomised studies in meta-analyses. 2013. Available at: http://www.ohri. ca/programs/ clinical _epidemio logy/oxford. htm. Accessed March 20, 2017.
- Guyatt GH, Oxman Andrew D, Vist Gunn E, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommend dations. BMJ 2008; 336:924-26.
- Jean K, Gabillard D, Moh R, et al. Effect of early antiretroviral therapy on sexual behaviors and HIV-1 transmission risk in adults with diverse heterosexual partnership status in Côte d’Ivoire. J Infect Dis 2013; 209:431-40.
- Jean K, Gabillard D, Moh R, et al. Decrease in sexual risk behaviours after early initiation of antiretroviral therapy: a 24-month prospective study in Côte d’Ivoire. J Int AIDS Soc 2014; 17:18977.
- The TEMPRANO ANRS 12136 Study Group. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808-22.
- The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795-807.
- Molina J, Grund B, Gordin F, et al. Who benefitted most from immediate treatment in START?A subgroup analysis [Abstract THAB0201]. XX International AIDS Conference, Durban, South Africa, July 21, 2016.
- Iwuji C, Orne-Gliemann J, Balestre E, et al. The impact of universal test and treat on HIV incidence in a rural South African population: ANRS 12249 TasP Trial, 2012-2016 [Abstract FRAC0105LB]. XX International AIDS Conference, Durban, South Africa, July 22, 2016.
- Jose S, Quinn K, Hill T, et al. Laboratory adverse events and discontinuation of therapy according to CD4+ cell count at the start of antiretroviral therapy. AIDS 2014; 28:1333-39.
- Le T, Wright E, Smith D, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368: 218-230.
- Okulicz JF, Le TD, Agan BK, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1einfected individuals. JAMA Intern Med 2015; 175:88– 89.
- Schneider G, Juday T, Wentworth III C, et al. Impact of health care payer type on HIV stage of illness at time of initiation of antiretroviral therapy in the USA. AIDS Care 2013; 25: 1470–76.
- Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiat ion of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–98.
- He N, Duan S, Ding Y, et al. Antiretroviral therapy reduces HIV transmission in discordant couples in rural Yunnan, China. PLoS One 2013; 8:e77981.
- CASCADE Collaboration. Short-term CD4 cell response after highly active antiretroviral therapy initiated at different times from seroconversion in 1500 seroconverters. J Acquir Immune Defic Syndr 2003; 32:303-10.
- CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med 2011; 171:1560–69.
- Gras L, Kesselring AM, Griffin JT, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defici Syndr 2007; 45:183–92.
- HIV CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24:123-37.
- HIV CAUSAL Collaboration. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med 2011; 154:509-15.
- Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–26.
- Merito M, Pezzotti P, ICONA Study Group. Comparing costs and effectiveness of different starting points for highly active antiretroviral therapy in HIV-positive patients. Eur J Health Econ 2006; 7:30–36.
- Palella FJ, Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med 2003; 138:620–26.
- Jia Z, Mao Y, Zhang F, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003-11): a national observational cohort study. Lancet 2013; 382:1195–1203.
- Sterne JA., Hernnr MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet 2005; 366:378–84.
- Lodi S, Phillips A, Logan R, et al. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV 2015; 2:e335–e3355.
- GarcGa F, De Lazzari E, Plana M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr 2004; 36:702–13.
- When to Start Consortium. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 2009; 373:1352–63.
- Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev 2013; (4):CD009153.
- Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830-89.
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493-505.
- Consortium WTS. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. The Lancet 2009; 373(9672):1352–1363.
- Hayes R, Ayles H, Beyers N, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment - a study protocol for a cluster randomised trial. Trials 2014; 15:57.
- Jain V, Byonanebye DM, Amanyire G, et al. Successful antiretroviral therapy delivery and retention in care among asymptomatic individuals with high CD4+ T-cell counts above 350 cells/μl in rural Uganda. AIDS 2014; 28:2241-49.