Information
Journal Policies
Comorbidity and Polypharmacy in Free-World and Incarcerated Hiv-Infected Patients: An Observational Study
Ana Belen Perona1,Jose Lorca2,Guillermo Telenti3,Sara Maestre4,Philip Wikman-Jorgensen5,Reyes Pascual6
2.Department Of Clinical Medicine. Universidad Miguel Hernández. Ctra N-332 S/N, CP 03500. San Juan De Alicante, Alicante, Spain.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose: To describe the comorbidities and drug prescriptions in a cohort of people living with HIV/AIDS (PLWHA).
Methods: Descriptive, observational, retrospective study of free-world and incarcerated PLWHA. Data were obtained from electronic and paper-based medical records. A standardized data recording process was used. Hypotheses were tested using the Chi-square test, Student´s T-test, or Mann-Whitney U-test, as appropriate.
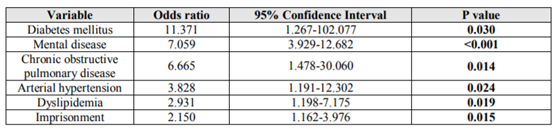
Findings: The study included 271 patients. Of those, 235 (87%) had one or more comorbidities, and the number of those increased with age. The mean daily pill burden was 5.4 (standard deviation [SD] 3.5). In our sample, 86.3% people were taking ≥ three pills and 53.9% ≥ five each day. The incarcerated patients (38.4% of the total) were younger than their free-world counterparts (43.82 years, SD 6.47 versus 50.41 years, SD 7.63; p < 0.001). In the free-world PLWHA, there was a higher percentage of hypertension (15.6% versus 2.9%), dyslipidemia (21.6% versus 7.7%), and cancer (11.4% versus 2.9%), while inmates had a higher prevalence of mental illness (61.5% versus 48.5%), hepatitis C virus (HCV) infection (87.5% versus 39.5%), and cirrhosis (16.3% versus 2.4%). In regression analysis the strongest associations were found for diabetes mellitus (OR 11.371; CI95% 1.267-102.077; p=0.03), mental disease (OR 7.059; CI95% 3.929-12.682; p˂0.001) and chronic obstructive pulmonary disease (OR 6.665; CI95% 1.478-30.06; p=0.014). It is also noteworthy that imprisonment was found associated with polypharmacy (OR 2.150; CI95% 1.162-3.976: p=0.015).
Originality/Value: Comorbidities and polypharmacy were frequent in our study. The pattern of comorbidities is very different between free-world and incarcerated PLWHA.
HAART; Toxicity; Treatment; HIV; Polypharmacy,AIDS
1. Introduction
There has been a notable increase in life expectancy in people living with HIV/AIDS (PLWHA) related to current antiretroviral therapies (ART).[1] Thus, this group of patients is increasingly older, with associated comorbidities and diverse concomitant treatments.[2,3] These comorbidities are more prevalent in PLWHA than in the general population.[4] They are associated with a prolonged period of immunosuppression as well as with inflammation, immune hyperactivation and the use of high doses of ritonavir. Mathematical models suggest that these comorbidities will only be more frequent in the future, as the age of patients infected with HIV increases.[5]The frequent polypharmacy in these patients due to their comorbidities is associated with a decrease in ART adherence;[6] the appearance of resistance, interactions, and toxicities;[7,8] and an increased risk of hospital admittance and death[9].
Incarcerated people have many differences compared to free-world patients. Incarcerated patients have a higher prevalence of HIV infection, hepatitis C virus (HCV), hepatitis B virus (HBV), and tuberculosis.[10]This higher prevalence is mainly attributed to the criminalization of drug use. Mental illness has also been reported to have a higher prevalence in incarcerated people than in free-world patients.[11] Research has mainly been focused on differences in the general population and scarce information exists about PLWHA. One report has described a higher prevalence of mental illness and substance abuse in incarcerated HIV-infected patients than among HIV-negative incarcerated patients.[12] However, it is unclear whether incarcerated PLWHA have different comorbidities compared to free-world PLWHA. As the comorbidities could be different, we could also expect differences in polypharmacy.
Understanding polypharmacy in patients on ART is a relevant topic to avoid possible interactions and toxicities and to analyze the impact that different treatments have on adherence and infection control. There is room for improvement in linkage to care after imprisonment and it has been shown that addressing comorbidities during imprisonment has the potential to increase long-term HIV-outcomes.[13,14] It is with this in mind that the present study has been conceived, with the primary aim of describing the most frequent comorbidities and prescription medications in a cohort of free-world and incarcerated PLWHA attended at the General University Hospital of Elda, Alicante, Spain. Our secondary aim is to describe the differences in comorbidity and polypharmacy among these two groups.
2. Materials And Methods
Observational, descriptive, retrospective study, analyzing the pharmacological treatments and comorbidities of PLWHA on ART, followed up as outpatients in Internal Medicine/Infectious Diseases of the General University Hospital of Elda. Around 300 PLWHA are treated in our outpatient clinics. All patients on follow-up during the study period were included. This hospital attends a health department of 200,000 inhabitants and is the second largest in the number of beds in the province of Alicante, Spain. In our outpatient clinics, we also attend the HIV-infected patients that are referred to our unit from the local prison. The patients are referred to our clinic at the discretion of the prison physician.
We collected data on the following variables: sex, birthdate, year of HIV diagnosis, risk factors in the acquisition of HIV, CD4, viral load, current ART, ART treatment experience, self-reported adherence (routinely documented in the medical record), concomitant treatments, type of prescription drugs, interactions with ART, daily pill burden, polypharmacy, comorbidities, illicit drug use.
We determined that comorbidities were present when these were included in the clinical history. We considered that patients were taking only the prescriptions that appeared on their clinical history. We defined polypharmacy as present in people taking five or more different pills per day, including ART. The database of the University of Liverpool was used to identify the contraindicated ART/non-ART interactions and those that require adjustments in dosage[15].
Data were collected in a consecutive sample of patients from June 1, 2014, to June 1, 2015. The sources of data used were patients’ paper-based clinical histories, the hospital’s electronic medical records program, “Hospital Discharge”, and the outpatient electronic medical records program, "ABUCASIS". When there was no record of comorbidities from those sources, we understood that none were present and that patients were not taking any other medication. We implemented a standardized protocol using a paper-based booklet of data collection forms; these data were then entered into an electronic database using the SPSS statistical package, v15.0 (SPSS Inc. Chicago, IL). We double-checked the data for consistency and gaps.
The STROBE statement was followed during the development of this article[16].
Statistical analysis was carried out using the SPSS 15 program (SPSS Inc. Chicago, IL). For the descriptive analysis, we used the usual parameters of mean and standard deviation (SD) for the quantitative variables with a normal distribution, and the median and interquartile range (IQR) for quantitative variables with a non-normal distribution. Qualitative variables are expressed as percentages. The comparison of quantitative variables was performed using the Student’s T-test or the Mann-Whitney U-test, depending on whether or not the data followed a normal distribution. We analyzed the qualitative variables using the Chi-square test, with Fisher’s correction when appropriate. P values of less than 0.05 were considered statistically significant. A logistic regression multivariate analysis was performed to identify factors associated with polypharmacy.
Patient data were anonymized to ensure confidentiality. The study was approved by the Ethics Committee of the Department of Health of Elda.
3. Results
A total of 271 patients were included in the study with a mean age of 47.88 years (SD 7.88, range 20 to 81); 82.3% were males. The percentage of patients over 50 was 31.6%, and over 60, 7.0%. Most (80.1%) had an undetectable viral load (< 20 copies/mL), and the median value of CD4 was 495.5 cells/mL (IQR 306 to 678). Of the total sample, 167 patients were free, and 104 were inmates at a correctional facility. The mean duration of HIV infection was 17.7 years (SD 7.38).
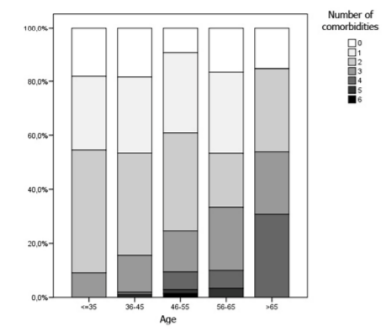
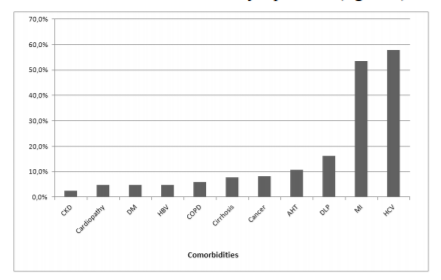
A total of 235 (87%) patients had one or more comorbidity, and the number of these increased with age, presenting a positive and significant correlation (r = 0.14, p = 0.021, figure 1). The most prevalent comorbidities were co-infection with hepatitis C virus (HCV), mental illness and dyslipidemia (figure 2).
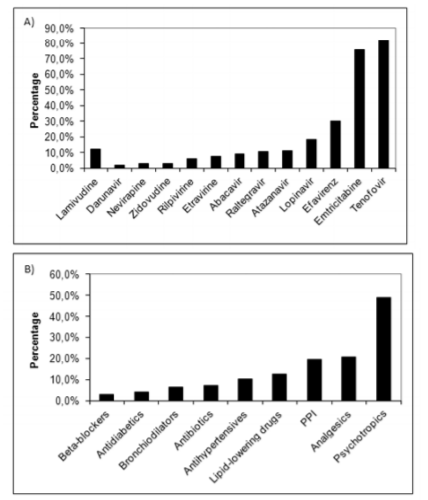
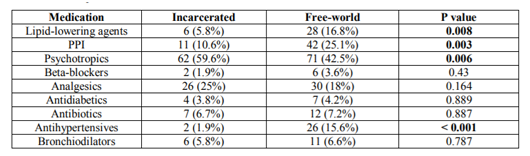
The mean daily pill burden was 5.4 (SD 3.5); 86.3% were taking three or more per day, and 53.9% were taking five or more. The most commonly prescribed drugs were psychotropics (n = 133, 49.1%), mostly benzodiazepines (n = 69, 25.5%), analgesics (n = 56, 20.7%), proton pump inhibitors (n = 53, 19.6%), antihypertensive drugs (n = 28, 10.3%), and lipid-lowering agents (n = 34, 12.5 %) (figure3).
The most commonly prescribed ART was TDF/FTC/EFV (figure 3), with 81.9% of the patients following a treatment regimen based on TDF. We identified interactions requiring dosage adjustments in 74 (27.3%) patients but no contraindicated combinations.
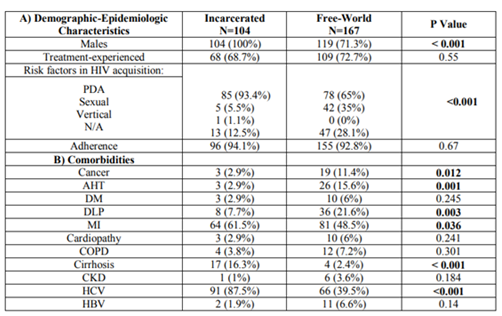
In our sample, 38.4% of the patients were inmates at a correctional facility, and this group was younger than their free-world counterparts (mean inmate age: 43.82 years, SD 6.47 versus mean free-world age: 50.41 years, SD 7.63; p < 0.001). The pattern of comorbidities was also different: free-world patients had a greater prevalence of hypertension (15.6% versus 2.9%; p=0.001), dyslipidemia (21.6% versus 7.7%; p=0.003), and cancer (11.4% versus 2.9%; p=0.012), while inmates were more likely to have mental illnesses (61.5% versus 48.5%; 0.036), HCV infection (87.5% versus 39.5%; p˂0.001) and cirrhosis of the liver (16.3% versus 2.4%; p˂0.001) (table 1).
The concomitant medications prescribed with ART (Table 2) were also different. There was a greater percentage of free-world patients taking proton pump inhibitors (25.1% versus 10.6%; p=0.003), antihypertensive drugs (15.6% versus 1.9%; p˂0.001), and lipid-lowering agents (16.8% versus 5.8%; p=0.008), and more inmates taking psychotropic medications (59.6% versus 42.5%; p=0.006).
The mean daily pill burden among incarcerated patients was higher than in the free-world group (6.2, SD 3.87 versus 4.9, SD 3.18; p = 0.004). However, the proportion of patients with drug interactions necessitating dosage adjustments was similar between the two (42.4% of inmates and 31.9% of free-world patients; p = 0.127).
A multivariate logistic regression analysis was undertaken and is presented in Table 3. It is noteworthy that the strongest associations were found for diabetes mellitus (OR 11.371; CI95% 1.267-102.077; p=0.03), mental disease (OR 7.059; CI95% 3.929-12.682; p˂0.001) and chronic obstructive pulmonary disease (OR 6.665; CI95% 1.478-30.06; p=0.014). It is also noteworthy that imprisonment was found associated with polypharmacy (OR 2.150; CI95% 1.162-3.976: p=0.015).
4. Discussion
In our study, we observed a high prevalence of comorbidities, with 87% of the patients having at least one apart from HIV. The most frequent comorbidities were HCV co-infection, mental illness, and dyslipidemia. These comorbidities have been reported as frequent comorbidities in PLWHA in other studies.(17,18) Likewise, over half of the patients had polypharmacy. The most frequent non-ART drug prescribed was a psychotropic drug, mainly benzodiazepines, reflecting the frequent association of HIV with mental disease.
The comorbidities associated with HIV infection and the potential drug interactions are different in different groups, as is clear from the variations observed between the group of patients who were detained in a correctional facility and those who were free. In the collective of free-world patients, the most frequent comorbidities were hypertension, dyslipidemia, and cancer (AIDS- and non-AIDS-defining cancers). Inmate patients, on the other hand, were more likely to have an HCV co-infection or a mental disorder. We believe that the higher age of the free-world population explains the increased prevalence of age-associated comorbidities. The patients at the correctional facility, on the other hand, presented more frequently with a history of parenteral drug use and therefore showed a higher prevalence of diseases associated with this behavior. The common co-existence of mental illness and HCV infection hints at possible interactions in this group of patients between the psychotropic drugs and the direct-acting antivirals (DAA) used to treat HCV.
Given the high prevalence of diagnosis of mental disorders and the frequent prescription of psychotropic drugs, efforts to tackle these diseases during imprisonment are noted and should lead to improved linkage to care after release.
Despite the fact that 82% of the patients were receiving antiretroviral regimens including tenofovir, the prevalence of kidney disease was very low, which suggests an adequate control and monitoring of renal function in our sample, although the mean age admittedly was under 50. As the population ages and the treatments continue, we will likely see an increase in the number of patients with renal deterioration.
The mean daily pill burden was 5.4 (SD 3.5). In other studies, the median daily pill burden oscillated between four and six, which is consistent with our series. Although most patients had polypharmacy, we did not find any contraindicated interactions in our patients’ prescriptions. However, it was necessary to adjust the dosages of different drugs in a third of our patients. This finding reflects the practices and the skills of the professionals responsible for caring for and prescribing treatments for PLWHA. Other studies have reported similar findings, with infrequent co-prescriptions of contraindicated drugs.[19] Nevertheless, the mean age of our cohort is relatively low, and the risk of interactions has been reported with greater frequency in patients of advanced age.[21] Green studied a cohort of patients over 60, finding a median daily pill burden of 13; 70% had at least one relevant interaction requiring consideration of a treatment modification, and in 11%, the interaction required avoidance of the combination. Also, 52% of the patients were taking one or more medications that were potentially inappropriate. In a study in 151 people with a similar mean age to our cohort, Zhou observed a burden of eight pills per day, with a significant association between the length of antiretroviral treatment and the presence of more than three comorbidities with the pill burden.[22] Given these findings, simplification strategies hold considerable interest, as does the use of combinations with low frequency of interactions, such as those based on integrase inhibitors[23-25].
The high percentage of free patients taking proton pump inhibitors is noteworthy. This proportion could be even higher, as patients frequently take omeprazole on their own and without telling their doctor. For this reason, it is necessary to take care in prescribing proton pump inhibitors in these patients, as they have been shown to interact with both ART and DAA for HCV[26-29].
In contrast to previous studies, we did not find age to have a statistically significant association with polypharmacy.[30] This finding could be because our population is relatively young, but also because we included disaggregated comorbidities in the regression analysis. Indicating that it is not the age per see that is associated with polypharmacy, but rather it is the different comorbidities that happen to be more prevalent at advanced ages.
Our research has the inherent limitations of a retrospective study, although we did consult diverse information sources to minimize the risk of missing data. In that sense, the low percentage of patients diagnosed with chronic obstructive pulmonary disorder (COPD) was noteworthy considering the high prevalence of smoking in our cohort. This disease may have been more present, but underdiagnosed, in our sample, and therefore it is not reflected in our data.
Moreover, there is a certain risk of selection bias in the group of incarcerated patients, as only a few PLWHA are referred to our center from the correctional facility, and one of the referral criteria is infection with HCV. This fact could have influenced the high prevalence of HCV infection that we observed in this group.
Despite the high frequency of polypharmacy, we did not detect problems with therapeutic adherence. This absence could be because adherence was measured by self-report, with all the limitations that imply. However, there was a high degree of virologic control, which does suggest that the patients were taking the prescribed treatment.
Finally, we note that the study was carried out in a single center, and its findings may not apply to other series in other centers. However, our results are consistent with other publications on Spanish and European series, with a high frequency of comorbidities, polypharmacy and potential drug interactions in PLWHA.[31] Furthermore, it is important to include the collective of incarcerated patients, which is a frequently neglected group that may present differential characteristics that are important to their management.
Our results allow us to conclude that both comorbidities and polypharmacy were very frequent in our population of PLWHA. Also, the pattern of comorbidities is very different in patients detained in correctional facilities, who have more mental illnesses and HCV infections, compared to free-world patients, who present more comorbidities associated with advancing age (hypertension, dyslipidemia, and cancer).
Acknowledgements
Dr.Vicente Gil for his generous collaboration.
Contributions Of Authors
AP: study design, data collection, and manuscript review; JL: data collection and manuscript review; GT: data collection and manuscript review; SM: data collection; PW: drafting of the manuscript and statistical analysis; RP: study design, manuscript review, supervision of study performance.
References
- The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008; 372(9635):293–9.
- Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: The swiss HIV cohort study. Clin Infect Dis. 2011; 53(11):1130–9.
- Marzolini C, Back D, Weber R, Furrer H, Cavassini M, Calmy A, et al. Ageing with HIV: Medication use and risk for potential drug-drug interactions. J Antimicrob Chemother. 2011; 66(9):2107–11.
- Schouten J, Wit FW, Stolte IG, Kootstra NA, Van Der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between hiv-infected and uninfected individuals: The age H IV cohort study. Clin Infect Dis. 2014; 59(12):1787–97.
- Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A V., et al. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis [Internet]. 2015; 15(7):810–8. Available from: http://dx.doi.org/ 10.1016/S1473-3099(15)00056-0
- Krentz HB, Gill MJ. The Impact of Non-Antiretroviral Polypharmacy on the Continuity of Antiretroviral Therapy (ART) Among HIV Patients. AIDS Patient Care STDS [Internet]. 2016 Jan [cited 2016 May 8]; 30(1):11–7. Available from: http://www.ncbi.nlm.nih.gov /pubmed/26544766
- Marzolini C, Elzi L, Gibbons S, Weber R, Fux C, Furrer H, et al. Prevalence of comedications and effect of potential drug-drug interactions in the Swiss HIV cohort study. Antivir Ther [Internet]. 2010; 15(3):413–23. Available from: http://www.scopus.com/inward/record.url?eid= 2-s2.0-77952923349&par -tner ID =40 &md5= 7b880fe787f6ae14c949a3afd6a09ddc
- Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf [Internet]. 2014; 13(1):59. Available from: http://www.pu bmedcentral.nih.gov/articlerender.fcgi?artid =3864987&tool=pmcentrez&render type=abstract
- Salazar J a, Poon I, Nair M. Clinical consequences of polypharmacy in elderly: expect the unexpected, think the unthinkable. Expert Opin Drug Saf. 2007; 6(May):695–704.
- Dolan K, Wirtz AL, Moazen B, Ndeffo-mbah M, Galvani A, Kinner SA, et al. Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. Lancet [Internet]. Elsevier Ltd; 2016;388(10049): 1089–102. Available from: http://linkinghub.elsevier.com /retrieve/pii/S0140673616304664
- Fazel S, Baillargeon J. The health of prisoners. Lancet [Internet]. Elsevier Ltd; 2011; 377 (9769):956–65. Available from: http://dx.doi. org/10.1016/S0140-6736(10)61053-7
- Zahari MM, Hwan Bae W, Zainal NZ, Habil H, Kamarulzaman A, Altice FL. Psychiatric and Substance Abuse Comorbidity among HIV Seropositive and HIV Seronegative Prisoners in Malaysia. am J Drug Alcohol Abuse [Internet]. Taylor & Francis; 2010 Jan 1; 36(1):31–8. Available from: https://doi.org/ 10.3109/0095 2990903544828
- Iroh PA, Mayo H, Nijhawan AE. The HIV care cascade before, during, and after incarceration: A systematic review and data synthesis. Am J Public Health. 2015; 105(7):e5–16.
- Loeliger KB, Altice FL, Desai MM, Ciarleglio MM, Gallagher C, Meyer JP. Predictors of linkage to HIV care and viral suppression after release from jails and prisons: A retrospective cohort study. Lancet HIV [Internet]. Elsevier Ltd; 2017; 3018(17):1–11. Available from: http://dx.doi.org/10.1016/S2352-3018(17)30209-6
- Liverpool U of. HIV Drug Interactions [Internet]. Available from: http://www.hiv-druginteractions.org/
- von Elm E, Altman DG, Egger M, Pocock SJ, G??tzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
- Niu L, Luo D, Liu Y, Silenzio VMB, Xiao S. The Mental Health of People Living with HIV in China , 1998 – 2014 : A Systematic Review. 2016;1998–2014.
- Bagheri Amiri F, Mostafavi E, Mirzazadeh A. HIV, HBV and HCV Coinfection Prevalence in Iran--A Systematic Review and Meta-Analysis. PLoS One [Internet]. 2016;11(3):e0151946. Available from: http://dx.doi.org/10.1371/journal.pone.0151946
- Acquah R, Graham H, Winter A. Quantifying polypharmacy in a large HIV-infected cohort. HIV Med. 2015; 16(9):583–4.
- Cantudo-Cuenca MR, Jimenez-Galan R, Almeida-Gonzalez C V, Morillo-Verdugo R. Concurrent use of comedications reduces adherence to antiretroviral therapy among HIV-infected patients. J Manag Care Pharm [Internet]. 2014;20(8):844–50. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CS C =Y&NEWS=N&PAGE=fulltext&D=emed12 & AN=2014501148%5Cnhttp://sfx.ucl.ac.uk/s fx_local?sid=OVID:embase&id=pmid:&id=doi :&issn=1083-4087&isbn=&volume= 20&issue =8&spage= 844&pages=84 4-850&da te=2014 &title=Journal+of+Managed+Ca
- Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc. 2014; 62(3):447–53.
- Zhou S, Martin K, Corbett A, Napravnik S, Eron J, Zhu Y, et al. Total daily pill burden in HIV-infected patients in the southern United States. AIDS Patient Care STDS. 2014; 28(6):311–7.
- Miller CD, El-Kholi R, Faragon JJ, Lodise TP. Prevalence and risk factors for clinically significant drug interactions with antiretroviral therapy. Pharmacotherapy [Internet]. 2007 Oct [cited 2016 Apr 16];27(10):1379–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17 896893
- Patel N, Abdelsayed S, Veve M, Miller CD. Predictors of clinically significant drug-drug interactions among patients treated with nonnucleoside reverse transcriptase inhibitor-, protease inhibitor-, and raltegravir-based antiretroviral regimens. Ann Pharmacother. 2011;45(3):317–24.
- Cervero M , Torres R , Jusdado JJ , Pastor S , Agud JL . [ Predictive factors of clinically significant drug -drug interactions among regimens based on protease inhibitors , non-nucleoside reverse transcriptase inhibitors and raltegravir]. Med clinicá [Internet]. 2016 Apr 15 [cited 2016 Apr 18]; 146(8):339–45. Available from: http://www.ncbi.nlm.nih.gov/ pubmed/26971988
- Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: An update. Drug Saf. 2014; 37(4):201–11.
- Arazo Garcés P, Los Santos Gil I De. Interacciones farmacocinéticas. Enferm Infecc Microbiol Clin [Internet]. Elsevier; 2013; 31(SUPPL.2):12–9. Available from: http:// dx.doi. org/10.1016/S0213-005X(13)70138-1
- Alsina MMP, Tuset Creus M, Miró JM. Farmacocinética e interacciones Del raltegravir. Enferm Infecc Microbiol Clin [Internet]. Elsevier; 2008;26(SUPPL. 12):23–8. Available from: http://dx.doi.org/10.1016 /S0213- 005X (08)76569-8
- Höner zu Siederdissen C, Maasoumy B, Marra F, Deterding K, Port K, Manns MP, et al. Drug-drug interactions with novel all oral interferon free antiviral agents in a large real-world cohort. Clin Infect Dis [Internet]. 2015;62(5):561–7. Available from: http://cid .oxfordjournals.org/lookup/doi/10.1093/cid/civ 973
- Moore HN, Mao L, Oramasionwu CU. Factors associated with polypharmacy and the prescription of multiple medications among persons living with HIV (PLWH) compared to non-PLWH. AIDS Care [Internet]. 2015 [cited 2016 Oct 5]; 27(12):1443–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2660848
- Iniesta-Navalon C, Franco-Miguel J, Gascon-Canovas J, Rentero-Redondo L. Identification of potential clinically significant drug interactions in HIV-infected patients: A comprehensive therapeutic approach. HIV Med. 2015; 16(5):273–9.