Information
Journal Policies
The Effect of Low-Dose (400mg) Versus Standard Dose Efavirenz (600mg) in HIV-Infected Adults: A Systematic Review and Meta-Analysis
Andrew Anglemyer1,Robert Castro2,Nathan Ford3,Hacsi Horvath4,George Rutherford5
2.School of Natural Sciences, California State University, Monterey Bay, Seaside, California, USA.
3.Department of HIV/AIDS, WHO, Geneva, Switzerland.
4.Global Health Sciences, University of California, San Francisco, San Francisco, California, USA.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objectives: Efavirenz (EFV) is available at a standard 600mg dose. However, a reduced 400mg dose has been suggested as a non-inferior alternative, perhaps lowering costs and risks of adverse events (AEs).
Methods:We searched published literature for randomized controlled trials (RCTs) and observational studies. Outcomes were viral load, change in CD4, and AEs.
Results: After 48 weeks, there was no difference in reduced viral load between low-dose and standard dose EFV in both RCTs and observational studies.
Conclusions : Furthermore, the reduced dose may decrease the risk of AEs. A reduced dose may be a safe cost-effective solution for HIV-infected populations in low-resource settings.
Efavirenz, treatment, standard dose,AIDS
1. Introduction
Efavirenz is a non-nucleoside reverse transciptase inhibitor, which is used as part of a three- or four-drug regimen to treat HIV infection. First licensed in 1998, it can be used both for initial therapy as well as for second-line therapy should resistance arise. An efavirenz-containing three-drug regimen with two nucleoside reverse transcriptase inhibitors – currently predominantly tenofovir disoproxil fumarate plus either emtricitabrine or lamivudine -- as a fixed-dose combination is recommended by the World Health Organization as the preferred initial regimen for antiretroviral therapy [1], and this regimen has been adopted by most countries [2]. Efavirenz can still be used safely in high-risk populations such as pregnant women with HIV and other adults with HIV taking rifampicin-based treatment for tuberculosis (TB) co-infection [3,4]. However, the drug is also associated with some adverse events and newer drugs have emerged with improved safety profiles [5]. As a result, there is a renewed interest in reducing the risk of efavirenz-related adverse events by reducing the dose. Efavirenz is currently available in a standard 600mg once-daily dose as part of a daily fixed dose combination. A 400mg dose as part of that fixed dose combination may be a non-inferior alternative, potentially reducing the Risk of Adverse Events As well As Costs [6].
2. Materials And Methods
The objective of this systematic review is to provide a summary of key evidence supporting the use of a reduced dose (400mg) of efavirenz and identify gaps where further research is required. Following a pre-defined protocol (available from the corresponding author), we searched Pub Med, Embassy, Cochrane Central Register of Controlled Trials, Web of Science for published literature and conference abstracts for randomized controlled trials and comparative observational studies. Screening and data extraction were done in duplicate (by authors AA and RC). We assessed risk of bias in each of the included published studies using a Risk of Bias tool, adapted from the Cochrane Collaboration. We used published estimated relative risks when provided and calculated the relative risks for dichotomous outcomes and mean differences with their 95% confidence intervals when necessary. Where appropriate, we pooled data from individual studies and summarized their effects. We determined between-study variation using the I2 statistic. We performed meta-analyses using a DerSimonian-Laird random-effects model. Clinical outcomes of interest included viral load, change in CD4 cell count, and adverse events. All analyses were performed in RevMan Version 5.3.
3. Results
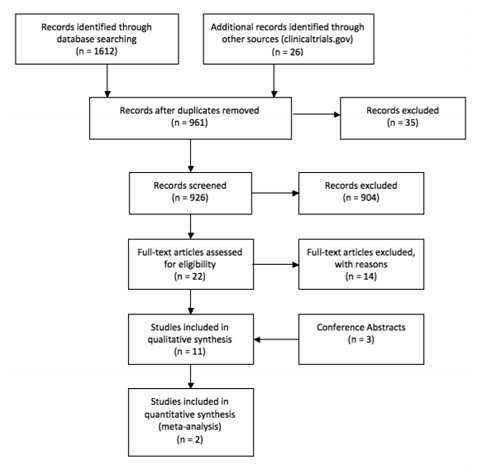
We identified 1,612 records through database searches of keywords, and 26 additional records through clinicaltrials.gov. After removing 677 clearly irrelevant studies and 36 duplicates, a total of 926 records were screened by AA and RC; GR adjudicated any disagreements about study selection. We excluded 904 records that did not meet our inclusion criteria, yielding 22 full-text articles assessed for eligibility. Fourteen were excluded for various reasons, including 9 because they were pharmacokinetic modeling studies, 1 was a validation assay study, 1 contained only patients on the reduced dose, 1 compared a group of patients who were given both 400 and 600mg dosages to a group with only 600mg, 1 was a feasibility study, and 1 compared a group with increasing dosages to a fixed dose group. An additional three conference abstracts from the HIV Drug Therapy Glasgow Congress 2014 and the Conference on Retroviruses and Opportunistic Infection (CROI) 2014 were included. The eight published articles [7-14] provided data from one randomized controlled trial (ENCORE1) and three observational studies. An additional conference abstract reported an analysis of observational data [data not shown], while two additional conference abstracts were identified but not included because they reported only early results of the ENCORE1 trial. All included studies contained only adult populations.
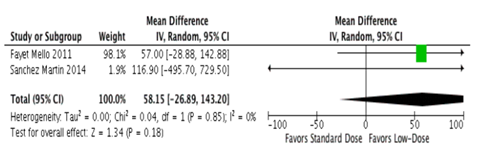
There was no difference in viral suppression as determined by the proportion of all included patients who were virally suppressed (< 200 copies/mL) at 48 weeks between low-dose and standard dose efavirenz in randomized controlled trials [8] (risk difference = -0.40%; 95% confidence interval (CI) -5.8% – 5.0%, p value = 0.88); this was supported by an observational study which found no difference in odds of achieving virological suppression (viral load < 50 copies/mL) at week 48 comparing standard and reduced dose efavirenz groups (odds ratio (OR) = 6.88; 95% CI 0.67-70.43, p value = 0.10) [11].Subgroup analyses by body mass index and ethnicity yielded no significant differences between doses [7]. Additionally, there was no difference in change in CD4 cell count in randomized controlled trials (mean difference = -5.0cells/µL (95% CI-39.0– 29.0 CD4 cells/µL; p value = 0.77) [8] and a synthesis of observational studies found similarly non-significant results (mean difference = 58.2 cells/µL (-26.9– 143.2; p value = 0.18) [9,10] (Figure 2). There was a reduced risk in efavirenz- associated adverse events among the lower-dose group when compared to the standard dose group (relative risk (RR) = 0.82; 95% Cl 0.69-0.98; p value = 0.03) .[8] Addition -ally, drug discontinuation due to drug-related causes was significantly less common among those who were treated with the lower dose efavirenz (RR = 0.45; 95% CI 0.26-0.80; p value = 0.01) [8],although this finding was only of borderline significance in the observational literature [11] (OR = 0.16; 95% CI 0.02-1.25; p value = 0.08).Resistance data were only reported in one study[8] and the authors reported resistance occurring in less than 3% of the participants. Additionally, they found no evidence that the resistance was related to any difference in treatment failure rates between the standard and low-dose EFV groups [8].
4. Discussion
Considering available data, we found no evidence to suggest a reduced dose of efavirenz is inferior to the standard dose regarding clinical outcomes. Moreover, relative to the standard dose of efavirenz the reduced dose may decrease the risk of adverse events. A reduced dose may be a safe cost-effective solution for HIV-infected populations in low-resource settings [15]. There is a need for further study into the efficacy of low-dose efavirenz in populations, notably HIV-infected pregnant women, people with TB co-infection, and long-term emergent drug resistance. These data support the World Health Organization recommendation to consider lower dose efavirenz as an alternative first-line agent. Trials are underway to assess the safety and efficacy in TB and pregnancy [15], and the results of these studies will inform the future role of efavirenz in first line antiretroviral therapy.
References
- World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Geneva, Switzerland: WHO; 2016.
- Nelson LJ, Beusenberg M, Habiyambere V,et al. Adoption of national recommendations related to use of antiretroviral therapy before and shortly following the launch of the 2013 WHO consolidated guidelines.AIDS 2014. 28(suppl 2):S217–24.
- Ford N, Mofenson L, Shubber Z, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis.AIDS 2014. 28(suppl 2):S123–31.
- Ford N, Shubber Z, Pozniak A, et al. Comparative safety and neuropsychiatric Adverse Events Associated with Efavirenz Use in First-Line Antiretroviral Therapy: A Systematic Review and Meta-Analysis of Randomized Trials.J Acquir Immune Defic Syndr 2015. 69 (4): 422-429.
- Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial.Lancet 2011. 378:229–237.
- Vitoria M, Hill A, Ford N, Doherty M, Khoo S, and Pozniak A. Choice of antiretroviral drugs for continued treatment scale-up in a public health approach: what more do we need to know? J Int AIDS Soc 2016. 19:20504.
- Amin J, Becker S, Belloso W, et al. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial.Lancet 2014. 383:1474-1482.
- Amin J, Becker S, Belloso W,et al.Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised,double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis 2015. 15:793-802.
- Fayet Mello A, Buclin T, Decosterd L, et al. Successful efavirenz dose reduction guided by therapeutic drug monitoring. Antiviral Therapy 2011. 16:189-197.
- Sánchez Martín A, Iglesias Gomez A, Garcia-Berrocal B, et al. Dose reduction of efavirenz: an observational study describing cost–effectiveness, pharmacokinetics and pharmacogenetics. Pharmacogenomics 2014. 15(7):997-1006.
- van Luin M, Gras L, Richter C, et al. Efavirenz dose reduction is safe in patients with high plasma concentrations and may prevent efavirenz discontinuations’ Acquir Immune Defic Syndr 2009. 52:240-245.
- Costa E, Biasi V, Concia E, et al. Budget impact analysis of efavirenz daily dose reduction at the Verona University Hospital. Le Infezioni in Medicina 2014. 2: 118-123.
- Dickinson L, Amin J, Else L, et al.ComprehensivePharmacokinetic,Pharmacodynamic and Pharmacogenetic Evaluation of Once -Daily Efavirenz 400 and 600 mg in Treatment -Na ̈ıve HIV -Infected Patients at 96 Weeks: Results of the ENCORE1 Study. Clin Pharmacokinet 2015.DOI: 10.1007/s40262-015-0360-5.
- Dickinson L, Amin J, Else L, et al.Pharmacokinetic and Pharmacodynamic Comparison of Once-Daily Efavirenz (400 mg vs. 600 mg) in Treatment-Na€ıve HIV-Infected Patients: Results of the ENCORE1 Study.