Information
Journal Policies
Biomarkers of Redox Balance in Tuberculosis - Human
Immunodeficiency Virus Co - Infected Cuban Patients
Lizette Gil-del Valle1,Alina Bareala Nunez2,Olga Pomier Suarez3,Rosario Gravier-Hernandez4,Yusimit Bermidez-Alfonso5,Dayme Hernandez-Requejo6,Teresa Rosell Guerra7,Maria Carla Hernandez Gonzalez-Abreu8
2.Medical Department, Hospital, Institute “Pedro Kourí” (IPK), La Habana, Cuba.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction: Human immunodeficiency virus (HIV) and Mycobacterium tuberculosis coinfection generates sustained inflammation with increased reactive oxygen species production. Pathogenic impact of systemic oxidative stress is recognized to influence on drug treatment and follow up. The aim of this case control study was to determine the redox status in Tuberculosis (TB)-HIV individuals and to explore relation between redox and HIV follow-up variables.
Methods: Blood samples were drawn from 75 individuals divided into three groups: HIV, TB-HIV and presumable healthy subjects. Redox, hematological, hemochemical, immunologic and virological indexes were determined.
Results: Relative to the control group, both HIV groups had significant differences in global indexes of damage and antioxidant status (p< 0,05). TB-HIV group showed a significantly higher damage (Total peroxide, advanced oxidation protein products) and lower antioxidant enzymatic activities compared to the control and HIV group´s (p< 0,05). Global modification of redox indexes showed 24 % of individuals with simultaneous detrimental differences related to HIV TB condition.
Conclusions: These results corroborate that oxidative stress occurs in HIV condition and also during TB-HIV co-infection with different molecular changes of follow-up indexes. Redox indexes diagnosis should be considering in early diagnosis and treatment of TB-HIV co-infection, which would be worthwhile to conduct a more comprehensive study and manage of infection.
HIV, aids, oxidative stress, Tuberculosis, Mycobacterium tuberculosis,AIDS
1. Introduction
The dire increase of infected people with Human immunodeficiency virus (HIV) worldwide is not limited to a particular racial group, country or community even though multidimensional efforts have been made to combat this disease [1-3]. Diverse authors have contributed to observations that reactive oxygen species (ROS) production are also increased during HIV infections and oxidative stress (OS) resulting could drive disulfide CD4 modification, necessary for HIV entry on host, CD4 T lymphocyte depletion and also viral replication causing a predisposition to opportunistic infections and malignancies [4-11].
OS occurs when there is a dysfunction in the overall balance between the production of ROS and the antioxidant defense mechanisms affecting redox circuits and modulating transcription factors or not influencing cellular survival, adaptation or death response [12-16].
Mycobacterium tuberculosis (Mtb) is a recognized facultative intracellular bacterium that replicates and persists within macrophages. To survive, the bacterium must sense and adapt to the oxidative conditions. Several antioxidant defenses including a thick cell wall, small molecule thiols, and protective enzymes are known to help the bacterium withstand OS. Mtb induces ROS production by activating both mononuclear and polymorphonuclear phagocytes which may promote tissue injury and inflammation in affected individual [17-20]. This further contributes to immune suppression, particularly in those with impaired antioxidant capacity, such as HIV infected patients [21-23].
Tuberculosis (TB) caused by Mtb infections remains a major health problem and the cases occur predominantly in the economically productive 15 - 49 years age group. TB is characterized by poor antioxidants defense that exposes to oxidative host tissue damage affecting organs function aggravating up condition [24-26]. It has been reported that the bactericidal potency of the myeloperoxidase-H2O2-halide system of neutrophilic granules exert bactericidal activities of the phagocytes that invariably produced increased ROS during phagocytic respiratory burst and it could produce significant reduction of enzymatic antioxidants (superoxide dismutase, catalase) and non-enzymatic antioxidants (glutathione) such as well as high malondialdehyde (MDA) concentrations suggesting increased generation of ROS due to lipid peroxidation which may contribute to the development of lung functional abnormalities [27-30].
In response to mycobacterial infection, parallel with ROS, another major antimicrobial pathway through inducible nitric oxide synthase (iNOS) leads to increased production of nitric oxide (NO), which further react with each other to produce highly ROS (peroxinitrite (OONO−). Bacterial pathogens as Mtb have been developed strategies to counteract production of host ROS, which interfere with the synthesis of these products, catabolize or repair the damage caused by them. A poorly understood aspect of Mtb is the exact mechanism of how it detects and evades the host immune system. But it is known that for survive inside macrophages; Mtb must withstand ROS produced by phagocyte oxidase (NOX2/gp91phox) and reactive nitrogen species produced by iNOS in the macrophage. The bacterium possesses several antioxidant defences including, protective enzymes such as catalase (KatG), superoxide dismutases (SodA and SodC), thioredoxins (Trx), peroxidase and peroxynitrite reductase complex (AhpC, AhpD, SucB, and Lpd), millimolar concentration of mycothiol and DNA-binding proteins such as Lrs2. Both reactivation of latent Mtb infection and progressive primary TB are substantially more common in HIV-1 infected subjects [31-35].
Like HIV infection, TB also has a long latency period with symptomatic presentation occurring from 3 months to decades after establishment of the infection. The only vaccine, developed more than a century ago, provides limited protection only during childhood. Curing TB requires prolonged combination of chemotherapy with several drugs mainly antibiotic [21]. Moreover, monitoring the success of therapy is questionable owing to the lack of reliable biomarkers. Although in developed countries, the rates of infection has fallen in the past century, the number is now again increasing which results in over 2000 deaths annually and an estimated 10 infections per 100,000 persons due to changes in social structures in cities, the HIV epidemic, and failure to improve treatment programs [3,15]. To change the situation, a detailed understanding of the crosstalk between human host and the pathogen Mtb is encouraged.
Patients with HIV-TB could present a range of presentations, including classical pulmonary TB, and various forms of extrapulmonary disease. Inappropriate treatment regimens and patient poor-compliance have led to the appearance of drug resistant TB [21].
Data that support the contribution of OS in TB-HIV pathogenesis has also been accrued at the cellular biochemical level [29,30, 36-38].
Inflammatory reactions combined with the disruption of the organism’s control mechanism could lead to a persistent pro-inflammatory state as, evidenced in a wide range of diseases that involve no-resolving or re-occurring reactivities [12,13]. Consistent changes in redox responsive cascades and in the expressions of corresponding target genes may have a similar or even greater impact on senescence as the direct radical inflicted damage of cellular constituents [15,16].
OS has been shown to enhance HIV replication, to induce the production of several inflammatory cytokines, to promote lymphocyte apoptosis and T cell dysfunction and could therefore contribute to increased viral replication and progression of immunodeficiency in patients dually infected with HIV and TB [4,11]
Gouripur et al and Madebo et al showed higher concentrations of MDA in co-infected patients respect HIV-seronegative TB patients, possibly reflecting increased OS in the former group. In addition ongoing TB infection has a greater impact on antioxidant status of HIV infection [29,39]. Madebo et al also reported that, concentrations of antioxidant vitamins and several thiol compounds were lower [39]. Awodele et al reported that there is lower antioxidant potential and higher lipid peroxidation in HIV-TB co-infected patients as compared to the HIV patients and the seronegative patients [36]. Considering backgrounds the aim of the study was to assess the redox status in HIV-TB Cuban individuals and compare to HIV positive patient and supposedly health volunteers. In addition follow up clinical biomarkers were evaluated. All data were statistical analyzed and relation between these variables was explored.
2. Experimental Procedure
A case control study was designed enrolling non HIV and unrelated HIV-aids positive individuals. All the patients were selected from the out-patients clinic at the Institute “Pedro Kourí” (IPK) Hospital for HIV. They all gave written informed consent to take part in the study after verbal and written explanation of the methods and risks involved were given. The work was developed by a multidisciplinary group, including clinical experts in HIV/aids management. Procedures were previously reviewed and approved by the Institute “Pedro Kourí” Committee for Research on Human Subjects considering one year for inclusion. The study is in accordance with the principle of the Declaration of Helsinki concerning the Ethical Principles for Medical Research Involving Human Subjects. The protocol was also approved by Determinant program of Cuban Ministry of Health (Code 151068).
Non probabilistic convenient sampling was used in according to the assistance of patients to the specialized consult in tertiary Hospital. The inclusion criteria for TB-HIV were typical pulmonary tuberculosis symptoms: 1- chest radiographs showing fibrous cavitary pulmonary infiltrates and 2- sputum specimen at least positive for acid-fast bacillus through Ziehl-Neelsen staining. The exclusion criteria were as follows: 1- smokers, 2- history of drug use (including vitamins, iron, and antibiotics), 3-blood transfusion history and no recent bleeding, 4- not pregnant and lactating, 5- not menstruating during blood acquisition, 6-previously receiving pulmonary tuberculosis treatment, and 7- patients with lung pathology. Seventy five subjects ranging from 30-50 years of age were enrolled sequentially. All subjects were assessed at the clinical visit. Anthropometry and laboratory tests were performed. Fifty subjects were HIV+, 25 were classified as aids and the others 25 were HIV with active pulmonary tuberculosis (TB-HIV) (confirmed TB and HIV test). Twenty five subjects were randomly selected age–matched healthy individual with serological evidences for HIV and/ or HCV negative infection and no abnormal laboratory values were finding.
Patients underwent a screening, which included the evaluation of their medical records, diet, and supplemental intake history, anthropometrics data (weight, height), and review of clinical lab results (complete blood count, glucose, creatinine, urea, liver enzymes). Demographic and age data were processed by SIDATRAT (software package 2008). Subjects were classified according to gender, age, ethnicity, viral load and CD4+ T lymphocyte subset count.
Viral load was determined with the Biomerieux polymerase chain reaction (PCR-NASBA) ultrasensitive assay with the lower limit of The ARV regimen consisted of a triple-drug combination allocated free, including two nucleoside reverse transcriptase inhibitors (IRT) and one protease inhibitors (PI), according to current guidelines were prescribed. The antiretroviral drugs used in the different combinations were prescribed daily at the following doses: RTIs zidovudine 600 mg, lamivudine 300 mg, didanosine 400 mg, PIs indinavir 2400 mg, ritonavir 1200 mg, saquinavir 2400 mg, nelfinavir 2250 mg. Patients also were oriented to use anti-tuberculosis drugs (rifampicin, isoniazid, etc) according ARV regimen established.
A study of T lymphocytes subsets CD3+/CD4+ in total blood was carried out. For each T lymphocyte subsets TM CD3 CD4 were used. These analyses were performed on a Cyflow Space Cytometer (PARTEC GmbH, Münster, Alemania) by FloMax 2014, Versión 2.9 program.
Viral load was determined with the Biomerieux polymerase chain reaction (PCR-NASBA) ultrasensitive assay with the lower limit of quantification of 50 IU.
Venous blood samples were taken from each fasted patient between 8.00 and 10.00 hours morning after informed consent was signed. Blood samples were collected by venipuncture into heparin-treated tubes and centrifuged to obtain serum.
For assay of SOD and CAT hemoglobin was extracted from haemolysate. For the rest of analysis, 3 mL of serum were employed. Serum samples were frozen at - 70°C and protected from light exposure until analyses were carried out.
All redox parameters were determined by spectrophotometric methods using an Ultrospect Plus Spectro-photometer from Pharmacia LKB
GSH from Sigma, St. Louis, M.O., USA was used to generate standard curves. Serum GSH concentrations were measured by the kinetics assay using the glutathione reductase reaction (40). Autoxidation of GSH to GSSG was prevented by addition of N-ethylmaleimide to the samples.
Malondialdehyde (MDA) concentrations were analyzed with the LPO-586 kit obtained from Calbiochem (La Jolla, C.A., USA). In this assay, stable chromophore production after 40 min of incubation at 45°C is measured at a wavelength of 586 nm by Pharmacia Spectrophotometer. To ensure that no lipid oxidation occurs during the assay, BHT [0.01% (v/v) of a 2% stock solution in ethanol] and EDTA (1 mM final concentration) were added to the sample prior to assay develop. Freshly prepared solutions of malondialdehyde bis [dimethyl acetal] (Sigma, St. Louis, M.O., USA) assayed under identical conditions were used as reference standards. Concentrations of MDA in serum samples were calculated using the corresponding standard curve and values were expressed as nmol g-1 Hb (41).
For the determination of the susceptibility to lipid peroxidation, serum samples were incubated with a solution of cupric sulfate (final concentration of 2 mM) at 37ºC for 24 h. The PP was calculated by subtracting the MDA concentration at time 0 from the one obtained at 24 h (42-43).
HPO was measured based on the oxidation of ferrous ions to ferric ions by hydroperoxides under acidic conditions. Ferric ions bind with the indicator dye xylenol orange (3,3'-bis(N,N-di(carboxymethyl)-aminomethyl)-o-cresolsulfone-phatein, sodium salt) to form a stable colored complex which can be measured at 560 nm (44).
SOD activities were assayed by a modified pyrogallol autoxidation method (45).
CAT activity was measured according with the method of Clairbone. Using a molar extinction coefficient of 43.6 M-1 cm-1, the rate of the first 30 s was used to calculate the activity. Catalase activity was expressed as U mg-1 Hb (46).
Serum AOPP was measured according to the methods of Witko-Sarsat et al [47]. The values were expressed in chloramine T equivalents and corrected by serum albumin concentrations.
Blood parameters such as hematocrit, hemoglobin, and erythrocyte sedimentation rate (ESR) were screened by Hematological counter MICROS 60. Others as triglycerides, creatinine, cholesterol, glucose, uric acid, urea, albumin, alkaline phosphatase, Gamma-Glutamyl (GGT), aspartate or alanine aminotransferase activity were performed by standard procedures in HITACHI analyzer 912, all in a specialized laboratory of IPK Hospital.
For descriptive statistics of continuous variables, means and standard deviations were calculated, whereas categorical variables were expressed as proportions. The normality of variables was evaluated by the Kolmogorov-Smirnov test. Comparisons between the cases and control groups were assessed using Kruskal-Wallis test followed by a post hoc Dunn's Multiple Comparison Test. Statistical significance was defined as p < 0,05. Pearson correlation coefficient were used to determine the relationship among the different parameters combining redox and follow up indexes. The SPSS software package version 20 and GraphPad Prism was used for all statistical analyses.
3. Results
The characteristics of the 75 subjects are showed in Table 1. The values of the 25 HIV-were considered as controls. There were no statistical significant differences between the groups according to demographics, gender, body mass index (BMI) and number of patients (p˃0.05). Major percent of patients in three groups were older than 38 years, skin color white and were on normal-weight.
Almost no patients had a previous history of opportunistic infections, except 7 patients whose previously presented wasting syndrome (loss of 10 % of weight in about 2 months), for 18 TB was the aids-defining illness [23].
The mean value of all biochemical, redox indexes and HIV progression markers evaluated for control and HIV groups are shown in Table 2 and 3.
The same characteristic were found on LDH, creatinine, albumin, uric acid, urea, glucose, cholesterol, triglycerides, ASAT and ALAT indexes values.
The ESR' mean values of HIV and TB HIV groups were significantly higher (p< 0.05) respect control group and it was out of the interval considered as physiological-reference despite not significant differences were found between them (p>0.05). GGT' and alkaline phosphatase mean value of TB HIV group was significant different respect HIV and control groups and it was out of interval considered as physiological-reference (Table 2).
PP is a global index. It assay serum antioxidant capacity shows serum susceptibility to lipid peroxidation. HIV and TB HIV patients had PP significantly higher value, suggesting reduced lipid-serum antioxidant capacity respect control value (p< 0.05). TB HV group PP wasn’t significant different respect HIV group (p0.05) (Table 3).
The immunologic and virological indexes not showed significant differences between HIV groups (p>0.05).
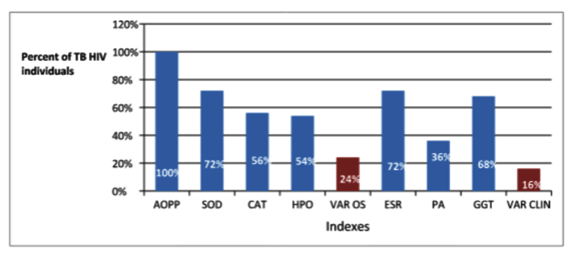
Simultaneous analyses of redox status permit to identify 8 patients with SOD, CAT, HPO and AOPP alterations which represent the 24 % of the TB HIV group. Five patients presented simultaneously alteration of ESR, ALP and GGT. When analyses of risk association were done oxidative damage and antioxidant depletion are related of TB HIV condition (Figure 1).
Correlation analysis showed significantly linear Pearson coefficient for ALP-ESR, GGT-VL, ESR-VL and VL-CD4 (p< 0.05) (Table 4). Positive correlations were found between ALP-ESR, GGT-VL, ESR-VL and negative for VL-CD4.
4. Discussion
Actually there are controversial data that substantiate the association of OS and persistent inflammation in several human diseases including HIV infection and co infected conditions [15,16]. It has been previously shown that the HIV-infected populations have significantly lower antioxidant concentrations than non HIV individuals [4,10,11]. Similar alteration occurred in patient with antiretroviral treatment and diverse clinical condition [7,8]. Altered redox metabolism may contribute to an amplify cellular damage resulting from generation of oxidized products, some of which are chemically reactive and lead to covalently modifying critical macromolecules [12,13].
In the present study, the concentrations of antioxidants in TB HIV coinfected patients were also low refereed to total antioxidant capacity and glutathione, lipid and protein oxidation indexes were augmented too in HIV groups and especially in TB HIV group. Between HIV groups differences in redox indexes arise similar to previous studies related to HIV TB coinfection [36,39]. Persistent OS have a dramatic impact on immunological, clinical and nutritional status in HIV infection [11].
Previous reports address that OS could be related to both, viral replication and also implicated on cell apoptosis in HIV infection. ROS could modulate and activate nuclear transcription factors, which ultimately lead to viral gene expression of HIV, and concomitant to HIV-related opportunistic infections or malignancies [5,16].
In the present study the reliable redox markers altered in TB HIV respect HIV condition were CAT and SOD activities and HPO and AOPP. CAT and SOD increased activities in HIV Tb patients differ from previous reports [29,36]. It is possible that differences are related to enzymatic determination on plasma what could indicate altered superoxide and peroxide generation related to HPO index [14]. Peroxide and superoxide have the ability to generate others reactive species by interacting with free transition metals producing oxidative tension on environment [16]. Depletion of GSH and antioxidant capacity in both groups evaluated as PP could be related to its consumption by increased chronicle generation of ROS. Respect hematologic and hemochemical indexes ESR, GGT and ALP showed alteration respect HIV patients. ESR is unspecific index attributed to infected condition in that case to TB HIV patients. ALP activity modification could be related to redox altered state with consequent activation or deactivation of different biomolecules by phosphorylation. GGT enzyme increased activation could also be related to pro oxidant condition, in which glutathione is used as cofactor for detoxification of ROS or conjugation reactions of different biomolecules [12].
The clinical outcome of HIV-1 infection contribute to exacerbate oxidative metabolism adding risk of molecular damage and also improving diverse virus replication or/and bacterial reproduction accruing poly pathology condition [11,23]. These findings could be explained in part by several mechanisms such as low intake of antioxidant or their precursors and mal absorption. Additional in peripheral tissues, enhanced cysteine metabolism with a consequent loss of sulphur-group may account for glutathione and antioxidant deficiency during HIV infection also persisting after antiretroviral therapy [8,[3] .
Abnormally high levels of prooxidant species as a consequence of chronic immune system activation by HIV infections could lead to a decline of antioxidants defense molecules and cumulative damage of cellular components generating augmented lipid peroxidation products, oxidized proteins and altered DNA sequences [11]. Almost redox implicated enzymes and molecules are physiologically endogenous generated and are involved in detoxification and general metabolism [13].
Correlation analysis considering follow up and redox indexes demonstrated linear interrelation between some parameters. This analysis offers an integral observation of multiple indicators. Redox metabolites and its species interact very quickly with biomolecules in cellular and fluid microenvironment and also with antioxidants so relation between different redox indicators could be multifactorial and nonlinear. Others indicators showed linear correlation which determine functional relations. In that sense interaction determine positive or negative coefficient [16]. Immune capacity diminish and viral load increase occurred in both HIV-TB and HIV group during oxidative condition.
Oxidative stress contribution evaluated as GGT increased activity could be related to modulation of transcription factors which in term influence on viral gene expression [48].
Taking into account that causes of polipathologies are complex and multifaceted, the recognition of molecular and cellular concert involved are crucial. A causal relationship between some elements such as oxidative macromolecules modifications, immunological status and viral load has emerged but the mechanism by which these molecular and biochemicals events occur remain to be established.
The OS evaluations will therefore become potential useful to characterize infection, antiviral combinations effects, as well as alternative therapies for counteracts oxidative damage [15].
Some contributions have been exploring mechanistic aspect of TB in vivo model for research comprehensive understanding of in vivo metabolism and repercussion of antioxidant on infection and cell proliferation [18,19,28]. Despite these concerns, substantial progress has been made toward an integrative understanding to delineate mechanism considering OS and prooxidant species as potential key participants in TB evolution [26,27]. In this sense significant difference evidenced in some redox indexes proved implications on HIV co infection evolution [22,29]. Gaining in knowledge of specific redox pathway and their relation to other factors, investigators will be provided with additional opportunities to impact both on quality of life and related diseases in humans and others species [15,38].
Several previous studies have been showed that also antiretroviral treatment has additional impact on pre-existing OS related to HIV condition.
Although huge beneficial impact of antiretroviral and antituberculosis treatment on morbi-mortality has been associated, a significant percentage of TB-HIV patients never achieve total remission [3,21, 34]. Understanding the interplay of bacterial and host factors in TB pathogenesis is critical for the rational effective intervention in future.
Considering previous elements some authors have been suggested and evaluate through redox indexes the use of antioxidants as agents which might reduce the incidence of OS as consequences of infection or treatment [49-54].
5. Conclusions
The present study contributes to evidences that OS evaluated in blood by several parameters could increase during TB HIV coinfection. It is suggested that cumulative damage reported had direct impact on functional efficiency of cell and tissues. Metabolic abnormalities as altered redox indexes remain an important part of complications in HIV infection and comorbidities. Their etiology, including roles for both non-HIV and HIV viral-related effects and treatment-associated factors, requires ongoing investigation. These complications could be involved in patients' active clinical status and long-term consequences. Management options are encouraged to clarified and evaluated therapeutically interventions effect which could provide substantial benefits to patients. These conclusions are also methodologically important for the follow-up and manage of infected individuals.
A clear understanding of the pathways most critically involved in TB progression and the consequences of altered cell behavior in the tissue micro-environments will provide nuggets of information which will help us in formulating better therapeutic approaches.
Altered redox status on TB could play a causal role in evolution by promoting damage to cell structure and functions and also redox driven process are stimulated modulating different stages of inflammation.
Acknowledgment
This work was partially supported by the Ministry of Public Health, Republic of Cuba (Project No. 151068). The authors gratefully thank to healthy volunteers and persons with HIV infection who enthusiastically participate in the study.
References
- Oguntibeju OO, van der Heever WMJ, Van Schalkwyk FM. Interplay between Socio-demographic variables, nutritional and immune status of HIV-positive/AIDS patients. Pak. J. Biol Sci. 2007; 10 (20): 3592 – 98.
- Oguntibeju OO, van der Heever WMJ, Van Schalkwyk FM. A review of the epidemiology, biology and pathogenesis of HIV. J. Biol. Sci 2007; 7(8): 1296-304.
- UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2016.
- Kashou A, Agarwal A. Oxidants and Antioxidants in the Pathogenesis of HIV/AIDS. The Open Reproductive Science Journal. 2011;3: 154-61.
- Buccigrossi V, Laudiero G, Nicastro E, Miele E, Esposito F. The HIV-1 transactivator factor (Tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS ONE 2011; 6(12): e29436. doi:10.1371/journal.pone.0029436.
- Cerutti N, Killick M, Jugnarain V, Papathanasopoulos M, Capovilla A. Disulfide reduction in CD4 Domain 1 or 2 is essential for interaction with HIV gp120, which impairs Thioredoxin-driven CD4 dimerization. J Biol Chem 2014; 289(15):10455-6.
- González I, Gravier R, Calas V, Reyes A, Pérez D, Hernández D, Bermúdez Y, Gil L, León OS. Oxidant/antioxidant status in subjects with HIV infection in different clinical conditions. Biomed Aging Pathol 2014, 4 (3):235-242, http://dx.doi.org/10.1016/j.biomag.2014.02.006 .
- Mandas A, Lorio E, Congiu M, Balestrieri C, Mereu A. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J Biomed Biotechnol. 2009:749-75.
- Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. 2013; 26:17–25.
- Mgbekem M, John M, Umoh I, Eyong E, Ukam N, Omotola B. Plasma Antioxidant Micronutrients and Oxidative Stress in People Living with HIV. Pakistan Journal of Nutrition. 2011; 10(3):214-9.
- Colado AN, Jacob V, Morimoto HK, Vissoci EM, Panis C. Redox-Driven Events in the Human Immunodeficiency Virus Type 1 (HIV- 1) Infection and their Clinical Implications. Current HIV Research 2015; 13: 143-50.
- Alfadda A, Sallam R. Reactive oxygen species in health and disease. J Biomed Biotechnol 2012:14.
- Valko M, Leibfritz D, Moncol J, Cronin M, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44-84.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Fourth Ed. Oxford University Press, 2007.
- Silvana A, Hepel M. Oxidative Stress: Diagnostics, Prevention, and Therapy. American Chemical Society 2011.
- Schieber M, Chandel N. ROS Function in Redox Signaling and Oxidative Stress. Current Biology. 2014; 24:453-62.
- Voskuil M, Bartek I, Visconti K, Schoolnik G. The response of mycobacterium tuberculosis to reactive oxygen and nitrogen species. Frontiers in microbiology 2011; 2:105.
- Elviro O. Mycobacterium tuberculosis host adaptation and evolution reflected by defense mechanisms against oxidative stress [PhD]: Universidad de Lisboa; 2012.
- Cappelli G, Volpe E, Grassi M, Liseo B, Colizzi V, Mariani F. Profiling of Mycobacterium tuberculosis gene expression during human macrophage infection: upregulation of the alternative sigma factor G, a group of transcriptional regulators, and proteins with unknown function. Research in microbiology 2006;157:445-55
- Brugarolas P, Movahedzadeh F, Wang Y, Zhang N, Bartek I, Gao Y, et al. The Oxidation-sensing Regulator (MosR) Is a New Redox dependent Transcription Factor in Mycobacterium tuberculosis. Journal of Biological Chemistry. 2012; 287(45):37704-12.
- Mulu A, Kassu A, Huruy K, Ameni G. Tuberculosis - Human Immunodeficiency Virus Coinfection: Bidirectional effect. Pharmacologyonline. 2008;2:301-18
- Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave E, et al. Preferential infection and depletion of Mycobacterium tuberculosis–specific CD4 T cells after HIV-1 infection. J Exp Med. 2010; 207:2869-81.
- Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature Age-Related Comorbidities Among HIV-Infected Persons Compared With the General Population. Clinical Infection Disease. 2011; 53(11):1120-6.
- Ramesh, Sudha K, Amareshwara M, Sameer, Rakesh M. Study of protein oxidation and antioxidants status in pulmonary tuberculosis patients. International Journal of Pharma and Bio Sciences. 2011; 2(3):104-9.
- Hashmi M, Ahsan B, Shah S, Khan M. Antioxidant Capacity and Lipid Peroxidation Product in Pulmonary Tuberculosis. Al Ame en J Med S c i 2012; 5(3):313 -9.
- Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn A. Redox homeostasis in mycobacteria: the key to tuberculosis control?: Cambridge University Press 2011. 39 p.
- Venketaraman V, Dayaram Y, Amin A, Ngo R, Green R, Talaue M, et al. Role of glutathione in macrophage control of Mycobacteria. Infect Immunity. 2003; 71(4):1864–71.
- Verma I, Jindal S, Ganguly N. Oxidative Stress in Tuberculosis. In: Ganguly N, editor. Studies on Respiratory Disorders, Oxidative Stress in Applied Basic Research and Clinical Practice. New York: Springer Science+Business Media; 2014. p. 101-14.
- Gouripur T, Desai P, Vani A, Gouripur K, Patil V. Comparison of lipid peroxidation product and enzymatic antioxidants in newly diagnosed pulmonary tuberculosis patients with and without human deficiency virus infection. Int J Pharm Bio Sci. 2012; 3(3):391 – 7.
- Guerra C, Morris D, Sipin A, Kung S, Franklin M, Gray D, et al. Glutathione and Adaptive Immune Responses against Mycobacterium tuberculosis Infection in Healthy and HIV Infected Individuals. PLoS ONE. 2011; 6(12):28378.
- Bartos M, Falkinham J, Pavlik I. Mycobacterial catalases, peroxidases, and superoxide dismutases and their effects on virulence and isoniazid-susceptibility in mycobacteria – a review. Vet Med. 2004; 49(5):161–70.
- Springer B, Master S, Sander P, Zahrt T, Mcfalone M, Song J, et al. Silencing of Oxidative Stress Response in Mycobacterium tuberculosis: Expression Patterns of ahpC in Virulent and Avirulent Strains and Effect of ahpC Inactivation. Infection and Immunity. 2001;39(10):5967–73.
- Mohod K, Dhok A, Kumar S. Status of oxidants and antioxidants in pulmonary tuberculosis with varying bacillary load. J Exp Sci 2011; 2(6):35–7.
- Butov D, Kuzhko M, Kuznetsova I, Grinishina O, Maksimenko O, Butova T, et al. Dynamics of Oxidant-antioxidant System in Patients with Multidrugresistant Tuberculosis Receiving Anti-mycobacterial Therapy. J Pulm Respir Med 2013; 3(5):1-3.
- Parchwani D, Singh S, Patel D. Total antioxidant status and lipid peroxides in patients with pulmonary tuberculosis. National Journal of Community Medicine 2011; 2(2):225-8.
- Awodele O, Olayemi S, Nwite J, Adeyemo T. Investigation of the levels of oxidative stress parameters in HIV and HIV-TB co-infected patients. J Infect Dev Ctries. 2012;6(1):79-85.
- Kaur K, Kishan J, Bedi GK, Ahi RS. Oxidants stress and antioxidants in pulmonary tuberculosis. Chest 2005;128: 3975
- Reddy YN, Murthy SV, Krishna DR, Prabhakar MC. Role of free radicals and antioxidants in tuberculosis patients. Indian Journal of Tuberculosis 2004; 51: 213–18.
- Madebo T, Lindtjorn B, Aukrust P, Berge R. Circulating antioxidants and lipid peroxidation products in untreated HIV tuberculosis patients in Ethiopia. Am J Clin Nutr. 2003; 78:117- 22.
- Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal Biochem 1974; 27:502–22.
- Erdelmeier I, Gerard D, Yadan JC, Chaudiere J. Reactions of N methyl-2-phenyl-indole with malondialdehyde and 4-hydroxialkenals. Mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem Res Toxicol 1998; 11:1184-94.
- Bartosz G. Total antioxidant capacity. Adv Clin Chem 2003; 37:219-92.
- Ozdemirler G, Mehmetcik G, Oztezcan S, Toker G, Sivas A, Uysal M. Peroxidation potential and antioxidant activity of serum in patients with diabetes mellitus and myocard infarction. Metab Res 1995; 271:194-6.
- Jiang Z, Woollard AC, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 1991; 26:853–6.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay form superoxide dismutase. Eur J Biochem 1974; 47:469–74.
- Clairborne A. Catalase activity. In: Green-Wald R, editor. Handbook of Methods for Oxygen Radical Research. Boca Ratón: CRC Press 1986. p. 283-4.
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguye-Knoa, Nguyen AT, Zingraff J, Jungers P, Deschamp-Latscha D. Advanced oxidation protein product as novel mediators of inflammation and monocytes activation in chronic renal failure. J Immunol 1998; 161:2524 -32.
- Mokondjimobe E, Longo-Mbenza B, Akiana J, Oswald Ndalla U, Dossou-Yovo R, Mboussa J, Parra HJ. Biomarkers of Oxidative Stress and PersonalizedTreatmentofPulmonary Tuberculosis: Emerging Role of Gamma- Glutamyltransferase. Advances in Pharmacological Sciences 2012, 2012: 465634 doi:10.1155/2012/465634
- Sevedrezazadeh E, Ostradrahini A, Mahboob S, Assadi Y, Ghaemmagami J, Poumogaddam M. Effect of vitamin E and selenium supplementation on oxidative stress status in pulmonary tuberculosis patients. Respirology 2008; 13:294–98.
- Dalvi S, Patil V, Ramraje N. The roles of glutathione, glutathione peroxidase, glutathione reductase and the carbonyl protein in pulmonary and extra pulmonary tuberculosis. J Clin Diagn Res. 2012; 6(9):1462–5.
- Pawar B, Suryakar A, Khandelwal A. Effect of micronutrients supplementation on oxidative stress and antioxidant status in pulmonary tuberculosis. Biomedical Research 2011;22 (4):455-9.
- Kondaveeti S, Annam V, Suresh D. Oxidative stress index as a novel biochemical marker in tuberculosis; with therapeutic benefit of antioxidant supplementation. BMC Infectious Diseases 2012; 12 (S1):66. doi:10.1186/1471-2334-12-S1-P66
- Saeidnia S, Abdollahi M. Role of micronutrients and natural antioxidants in fighting against HIV; a quick mini-review. Res J Pharmacognosy RJO 2014; 1 (4):49-55.
- Agarwal A, Prasad R, Jain A. Effect of green tea extract (catechins) in reducing oxidative stress seen in patients of pulmonary tuberculosis on DOTS Cat I regimen. Phytomedicine 2010; 17(1): 23–27.